机器学习引导下发现SLC25A45作为线粒体甲基化氨基酸输入和肉毒碱合成的中介
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
溶质载体(SLCs)通过跨膜运输小分子和离子来调节细胞和生物体的代谢,但约20%的生理底物仍然难以捉摸。为了解决这个问题,我们开发了一个机器学习平台来预测基因代谢物的关联。该方法鉴定出UNC93A和SLC45A4分别是乙酰氨基葡萄糖和多胺的候选质膜转运蛋白。此外,我们发现SLC25A45作为线粒体转运体与血清甲基化碱性氨基酸水平相关,碱性氨基酸是蛋白质分解代谢的产物。从机制上说,SLC25A45对于线粒体进口甲基化的碱性氨基酸是必要的,包括ADMA和TML,后者作为肉毒碱合成的前体。与这一观察结果一致,SLC25A45的缺失会损害肉毒碱的合成,并在禁食条件下减弱含肉毒碱代谢物的上调。通过促进线粒体TML的输入,SLC25A45将蛋白质分解代谢与肉毒碱的产生联系起来,在禁食期间维持β氧化。总之,我们的研究确定了三种slc的假定底物,并为转运体去孤儿化提供了资源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
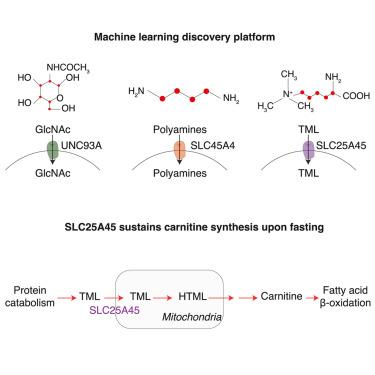
Machine-learning-guided discovery of SLC25A45 as a mediator of mitochondrial methylated amino acid import and carnitine synthesis
Solute carriers (SLCs) regulate cellular and organismal metabolism by transporting small molecules and ions across membranes, yet the physiological substrates of ∼20% remain elusive. To address this, we developed a machine-learning platform to predict gene-metabolite associations. This approach identifies UNC93A and SLC45A4 as candidate plasma membrane transporters for acetylglucosamine and polyamines, respectively. Additionally, we uncover SLC25A45 as a mitochondrial transporter linked to serum levels of methylated basic amino acids, products of protein catabolism. Mechanistically, SLC25A45 is necessary for the mitochondrial import of methylated basic amino acids, including ADMA and TML, the latter serving as a precursor for carnitine synthesis. In line with this observation, SLC25A45 loss impairs carnitine synthesis and blunts upregulation of carnitine-containing metabolites under fasted conditions. By facilitating mitochondrial TML import, SLC25A45 connects protein catabolism to carnitine production, sustaining β-oxidation during fasting. Altogether, our study identifies putative substrates for three SLCs and provides a resource for transporter deorphanization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


