利用阳离子辅助脂质和蛋白质冠的聚合脂质纳米颗粒用于肺靶向递送一种新型抗癌药物。
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
肺癌仍然是世界范围内癌症相关死亡的主要原因之一,这突出表明迫切需要更有效的治疗策略。纳米医学提供了一种很有前途的途径,可以通过在肺部局部给药来改善治疗结果。从最近mRNA脂质纳米颗粒的成功中获得灵感,我们开发了一种新型的聚合物-脂质纳米颗粒(P-LNPs),设计用于封装RB-012, RB-012是一种抑制14-3-3蛋白功能的抗癌化合物,但由于其阳离子和两亲性而迅速从体循环中清除。RB-012与阴离子聚合物聚丙烯酸(PAA)以及胆固醇、聚乙二醇化和带电的辅助脂质的各种组合共同组装,形成稳定的P-LNPs,显著阻碍体外药物过早释放。这种方法导致Sprague-Dawley大鼠静脉给药(2 mg/kg)后生物利用度增加了30倍。通过加入16-32 摩尔%的阳离子脂质DOTAP来改变辅助脂质组成,与未配制的RB-012相比,肺部药物暴露增加了 > 50倍。这些生物分布的增强与纳米颗粒表面蛋白质电晕谱的改变有关,与阴离子辅助脂质(DOPE)制备的P-LNPs相比,与DOTAP配制的P-LNPs以浓度依赖的方式增加了蛋白质电晕的吸附程度。体外和卵内实验证实,P-LNPs显著提高了RB-012的抗肿瘤功效,支持其作为肺癌靶向治疗平台的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
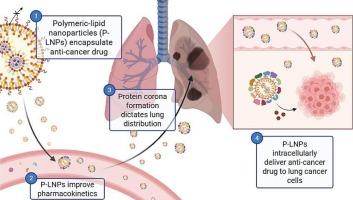
Polymeric-lipid nanoparticles that leverage cationic helper lipids and the protein corona for lung-targeted delivery of a novel anti-cancer drug
Lung cancer remains one of the leading causes of cancer-related mortality worldwide, highlighting the urgent need for more effective therapeutic strategies. Nanomedicine offers a promising avenue to improve treatment outcomes by enabling localised drug delivery within the lungs. Drawing inspiration from the recent success of mRNA lipid nanoparticles, we developed a novel class of polymeric-lipid nanoparticles (P-LNPs) designed to encapsulate RB-012, an anticancer compound that inhibits 14–3-3 protein function but is rapidly cleared from systemic circulation due to its cationic and amphiphilic properties. RB-012 was co-assembled with the anionic polymer polyacrylic acid (PAA) and various combinations of cholesterol, pegylated, and charged helper lipids to form stable P-LNPs that significantly impeded in vitro premature drug release. This approach resulted in >30-fold increase in bioavailability following intravenous administration (2 mg/kg) to Sprague-Dawley rats. Varying the helper lipid composition, through the inclusion of 16–32 mol% of the cationic lipid, DOTAP, yielded a > 50-fold increase in pulmonary drug exposure compared to unformulated RB-012. These biodistribution enhancements were linked to altered protein corona profiles on the nanoparticle surface, with P-LNPs formulated with DOTAP increasing the degree of protein corona adsorption in a concentration-dependent manner, compared to P-LNPs prepared with the anionic helper lipid, DOPE. In vitro and in ovo assays confirmed that the P-LNPs significantly improved the anti-tumour efficacy of RB-012, supporting their potential as a targeted therapeutic platform for lung cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


