基于芳基嘌呤的丝霉素模拟物作为寨卡病毒甲基转移酶抑制剂的设计、合成和评价。
IF 4.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
以寨卡病毒(ZIKV)甲基转移酶(MTase)的SAM/SAH结合位点为靶点,设计合成了模拟sininefungin的芳嘌呤衍生物。这些化合物含有腺嘌呤或6-甲基-7-去氮杂嘌呤碱基,而丝霉素的核糖已被苯胺取代,通过CO或CH2单元与其氨基酸链相连。化合物18、29和31抑制ZIKV 2′-O-MTase活性。对接研究表明,化合物18和29与嘌呤和氨基酸结合位点相互作用,有效地模拟了sininefungin。相反,化合物31的氨基酸链位于核糖结合位点的上方。值得注意的是,化合物18对寨卡病毒表现出适度的抗病毒活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
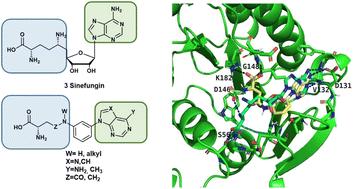
Design, synthesis and evaluation of arylpurine-based sinefungin mimetics as zika virus methyltransferase inhibitors
Arylpurine derivatives were designed and synthesized to mimic sinefungin by targeting the SAM/SAH binding site of zika virus (ZIKV) methyltransferase (MTase). These compounds incorporate adenine or 6-methyl-7-deazapurine bases, while the ribose of sinefungin has been replaced by an aniline, linked to its amino acid chain via a CO or a CH2 unit. Compounds 18, 29 and 31 inhibited ZIKV 2′-O-MTase activity. Docking studies showed that compounds 18 and 29 interact with both the purine and amino acid binding sites, effectively mimicking sinefungin. In contrast, compound 31 has its amino acid chain positioned above the ribose binding site. Notably, compound 18 exhibited modest antiviral activity against ZIKV.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


