黄柏碱通过抑制p38 MAPK-p47phox途径介导的中性粒细胞胞外陷阱的形成和ROS的产生来缓解急性胰腺炎
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
背景:急性胰腺炎(AP)是胰腺的一种炎症性疾病,其中中性粒细胞胞外陷阱(NETs)的形成在其发病机制中起着至关重要的作用。本研究通过研究黄柏衍生生物碱黄柏碱(phlodendrine, PHE)对AP的治疗作用,探讨了NETs的形成及其机制。方法分离小鼠骨髓中性粒细胞,体外刺激形成NETs,评价PHE的作用。建立caerulein诱导的AP小鼠模型,以评估PHE的体内疗效。利用网络药理学和RNA-seq分析来研究PHE减轻AP的机制。使用p38抑制剂验证了鉴定的靶点的功能作用。结果sphe能明显抑制体外NETs的形成和活性氧(ROS)的产生。体内实验进一步发现,PHE治疗可减轻胰腺损伤和炎症,并伴有AP小鼠NETs形成和中性粒细胞浸润减少。从机制上看,PHE靶向p38 MAPK通路,抑制其激活和随后的p47phox膜易位。此外,与p38抑制剂共给药可消除PHE对NETs形成和ROS产生的抑制作用。结论phe通过靶向p38 MAPK-p47phox通路,抑制NETs的形成和ROS的产生,减轻AP的胰腺损伤和炎症。这表明它有潜力成为治疗AP的创新治疗剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
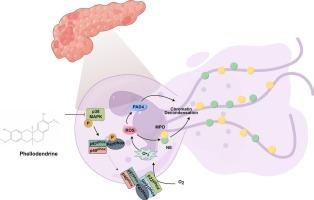
Phellodendrine alleviates acute pancreatitis by inhibiting p38 MAPK-p47phox pathway-mediated neutrophil extracellular traps formation and ROS production
Background
Acute pancreatitis (AP) is an inflammatory disorder of pancreas, where the formation of neutrophil extracellular traps (NETs) plays a crucial role in its pathogenesis. This study examined the therapeutic effect of Phellodendrine (PHE), a Phellodendron-derived alkaloid, on AP by assessing NETs formation and underlying mechanisms.
Methods
Mouse bone marrow neutrophils were isolated and stimulated to form NETs in vitro to assess PHE's impact. A Caerulein-induced AP mouse model was developed to assess the in vivo efficacy of PHE. Network pharmacology and RNA-seq analysis were utilized to investigate the mechanisms through which PHE alleviates AP. The functional role of the identified target was verified using a p38 inhibitor.
Results
PHE markedly inhibited NETs formation and reactive oxygen species (ROS) generation in vitro. In vivo experiments further revealed PHE treatment alleviated pancreatic injury and inflammation, which was accompanied by reduced NETs formation and neutrophil infiltration in the mouse of AP. Mechanistically, PHE targeted the p38 MAPK pathway, suppressing its activation and the subsequent membrane translocation of p47phox. Furthermore, co-administration with a p38 inhibitor abolished the inhibitory effects of PHE on NETs formation and ROS production.
Conclusion
PHE mitigates pancreatic injury and inflammation in AP through its inhibitory effect on NETs formation and ROS production, mediated by targeting the p38 MAPK-p47phox pathway. This indicates its potential as an innovative therapeutic agent for the treatment of AP.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


