钌催化叠氮化物-硒代炔环加成:区域选择性范围、机理和来源的合成-计算联合研究
IF 4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
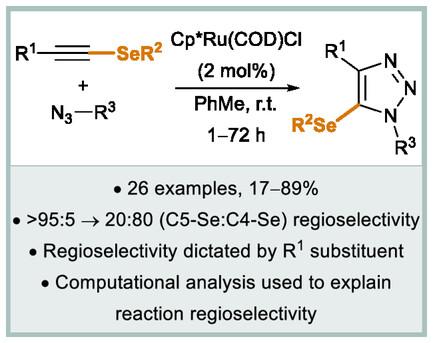
报道了一种钌催化的叠氮化物-炔环加成反应(RuAAC),其中含有硒代炔,用于合成硒取代的1,2,3-三唑产品(26例,产率高达89%)。该反应与具有供电子和吸电子基团的烷基和芳基叠氮化物反应良好。这对比了使用规则端炔和内炔的RuAAC反应,其中芳基叠氮化物含吸电子基团通常是不耐受的。环加成的区域选择性高度依赖于炔的非硒取代基的性质。这对涉及硫代炔的RuAAC反应的早期报告提出了挑战,并对这些反应的区域选择性的认知提出了质疑。计算模型的反应分布为四炔底物提供了对比区域选择性准确再现实验结果。所观察到的区域选择性主要归因于炔碳与钌配位后的相对亲核性,并提出了一种预测反应区域选择性的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ruthenium-Catalyzed Azide-Selenoalkyne Cycloadditions: A Combined Synthetic–Computational Study into Reaction Scope, Mechanism, and Origins of Regioselectivity
A ruthenium-catalyzed azide–alkyne cycloaddition (RuAAC) reaction involving selenoalkynes is reported for the synthesis of selenium-substituted 1,2,3-triazole products (26 examples, up to 89% yield). The reaction works well with a wide range of alkyl and aryl azides bearing both electron-donating and -withdrawing groups. This contrasts RuAAC reactions using regular terminal and internal alkynes, where aryl azides bearing electron-withdrawing groups are not generally tolerated. The regioselectivity of the cycloaddition is highly dependent on the identity of the non-selenium substituent of the alkyne. This challenges earlier reports on RuAAC reactions involving thioalkynes and questions the perceived understanding of the regioselectivity of these reactions. Computational modeling of the reaction profiles for four alkyne substrates that provided contrasting regioselectivities accurately reproduces the experimental results. The observed regioselectivity is primarily attributed to the relative nucleophilicity of the alkyne carbons upon coordination to ruthenium and an approach is proposed in which reaction regioselectivity may be predicted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


