p75神经营养因子受体通过感觉神经支配控制骨骼干细胞生态位
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
低骨量在阿尔茨海默病(AD)中经常观察到,但其潜在机制仍然知之甚少。在这项研究中,我们证明了感觉神经构成了骨骼干细胞(SSC)生态位的关键组成部分。神经营养因子受体p75NTR在神经元或感觉特异性细胞中缺失,但在成骨细胞或交感细胞中没有缺失,导致感觉神经支配减少,SSC稳态破坏,以及明显的骨质流失。尽管不能排除p75NTR在SSC中的细胞内在作用,但进一步涉及感觉去神经支配或移植到感觉神经元特异性p75NTR缺乏的宿主的实验证实SSC成骨受损。在机制上,p75NTR控制神经元骨桥蛋白(SPP1)的表达,进而促进SSC自我更新和成骨分化。值得注意的是,在AD小鼠模型中发现p75NTR-SPP1信号轴被破坏,这为AD相关的骨质减少提供了直接的机制解释,并强调了靶向神经控制ssc的治疗潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
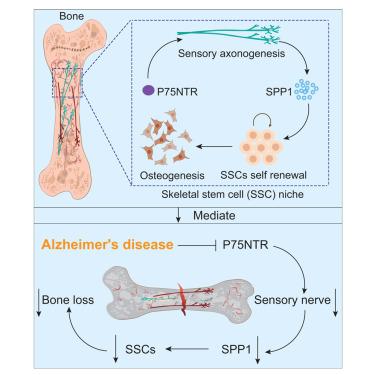
The p75 neurotrophin receptor controls the skeletal stem cell niche through sensory innervation
Low bone mass is frequently observed in Alzheimer’s disease (AD), yet the underlying mechanisms remain poorly understood. In this study, we demonstrate that sensory nerves constitute a critical component of the skeletal stem cell (SSC) niche. Deletion of the neurotrophin receptor p75NTR in neurons or sensory-specific cells, but not in osteogenic or sympathetic cells, resulted in reduced sensory innervation, disrupted SSC homeostasis, and significant bone loss. Although a cell-intrinsic role of p75NTR in SSCs cannot be ruled out, further experiments involving sensory denervation or transplantation into hosts with sensory-neuron-specific p75NTR deficiency confirmed impaired SSC osteogenesis. Mechanistically, p75NTR controls the expression of neuronal osteopontin (SPP1), which in turn promotes SSC self-renewal and osteogenic differentiation. Notably, this p75NTR-SPP1 signaling axis was found to be disrupted in AD mouse models, offering a direct mechanistic explanation for AD-associated osteopenia and highlighting the therapeutic potential of targeting neural control of SSCs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


