H2O2蚀刻α-MnO2调控甲醛氧化途径:氧空位的作用
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
α-MnO2的工程氧空位是生成活性氧以提高HCHO氧化催化性能的有效策略。在本研究中,通过高锰酸钾(KMnO4)与甲醇的原位氧化还原反应合成了棒状α-MnO2,然后通过调整H2O2蚀刻时间来调节表面氧空位。蚀刻时间是催化增强的关键因素,α-MnO2-E-2(2 h蚀刻)在60 °C下表现出优异的活性、稳定的稳定性和完全的HCHO转化。表征表明,通过改变H2O2蚀刻时间,可以有效地调节催化剂表面的氧空位。α-MnO2-E-2表现出最高的(Mn2+ + Mn3+)/Mntotal比率和最低的平均氧化态(AOS),这与最大的氧空位形成直接相关。氧空位的引入促进了氧的迁移和活化,从而加速了HCHO的氧化。原位漂移分析进一步证实,α-MnO2-E-2的中间产物只有甲酸盐,表明H2O2蚀刻将反应途径简化为HCHO→HCOO→H2O + CO2。本文章由计算机程序翻译,如有差异,请以英文原文为准。
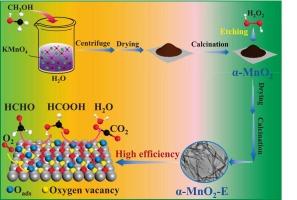
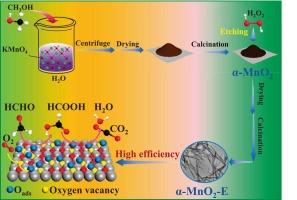
Modulating the formaldehyde oxidation pathway via H2O2 etching on α-MnO2: the role of oxygen vacancies
Engineering oxygen vacancies in α-MnO2 represents an effective strategy for generating active oxygen species to enhance catalytic performance in HCHO oxidation. In this study, rod-like α-MnO2 was synthesized via an in situ redox reaction between potassium permanganate (KMnO4) and methanol, followed by modulation of surface oxygen vacancies through the adjustment of H2O2 etching time. Etching duration critically governed catalytic enhancement, with α-MnO2-E-2 (2 h etching) exhibiting exceptional activity, robust stability and complete HCHO conversion at 60 °C. Characterization revealed oxygen vacancies on the catalyst surface could be effectively regulated by modifying the H2O2 etching time. Significantly, α-MnO2-E-2 demonstrated the highest (Mn2+ + Mn3+)/Mntotal ratio and lowest average oxidation state (AOS), directly correlating with maximized oxygen vacancy formation. The introduction of oxygen vacancies facilitated superior oxygen mobility and activation, thereby accelerating HCHO oxidation. In situ DRIFTS analysis further confirmed that only formate species were observed as intermediates on α-MnO2-E-2, demonstrating that H2O2 etching simplified the reaction pathway to HCHO → HCOO− → H2O + CO2.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


