氨基氧基介导的电催化氧化功能化反应:用苯胺制备n -苄基苯胺
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了一种用氨基氧基催化反应从胺中制备亚胺的方法。它是在商用电化学设备中使用现成的电极进行的,不需要使用参比电极。该方法在温和的条件下进行,并在一系列结构多样的苯胺中提供良好到优异的分离产率。选择性高,获得亚胺优于更常见的腈,并且该过程易于扩展到克水平。操作简单,反应时间短,使该方法成为有机化学家工具箱中有价值的补充。本文章由计算机程序翻译,如有差异,请以英文原文为准。
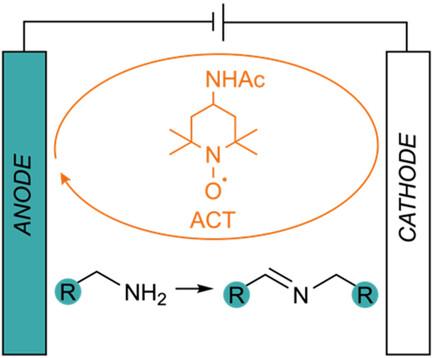
Oxidative Functionalization of Amines Using an Electrocatalytic Aminoxyl-Mediated Reaction: Preparation of N-Benzylidenebenzylamines from Benzylamines
A methodology is reported for the preparation of imines from amines by means of an electrocatalytic aminoxyl-mediated reaction. It is performed using readily available electrodes in a commercially available electrochemistry apparatus and does not require the use of a reference electrode. The methodology proceeds under mild conditions and delivers good to excellent isolated yields across a range of structurally diverse benzyl amines. Selectivity is high, with imines obtained in preference to the more commonly reported nitriles, and the process is readily scalable to the gram level. Operational simplicity and short reaction times make this method a valuable addition to the organic chemist's toolkit.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


