钯(II)催化远端C?8-氨基喹啉酰胺的H功能化:三芳基甲烷衍生物的简易合成
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文报道了一种用对醌方法催化8-氨基喹啉衍生物的钯催化氧化远端C - H烷基化的直接方法。该方法实现了喹啉的选择性c5烷基化,并表现出与传统钯催化的sp2 C - H活化不同的位点选择性。该反应通过螯合诱导的C - H远程官能团化进行,广泛的喹啉胺类化合物成功地与对醌类化合物偶联,表现出良好的底物范围和官能团耐受性。初步的机理研究表明,可逆的C - H金属化步骤是催化循环的一部分。本文章由计算机程序翻译,如有差异,请以英文原文为准。
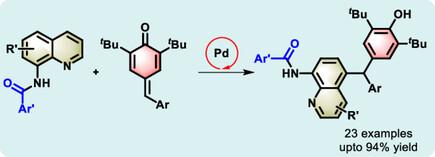
Palladium(II)-Catalyzed Distal C?H Functionalization of 8-Aminoquinolinamides: Facile Synthesis of Triarylmethane Derivatives
Herein, a straightforward approach is reported for the palladium-catalyzed oxidative remote CH alkylation of 8-aminoquinoline derivatives using para-quinone methides. This method enables selective C5-alkylation of quinolines and exhibits a site-selectivity distinct from that typically observed in convetional palladium-catalyzed sp2 CH activation. The reaction proceeds via chelation-induced remote CH functionalization, and a broad range of quinolinamides are successfully coupled with p-quinone methides, demonstrating excellent substrate scope and functional group tolerance. Preliminary mechanistic studies suggest a reversible CH metalation step as part of the catalytic cycle.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


