丙胺与异氰酸甲酯及水的三组分Van Leusen反应模块化合成3,4-二取代吡咯
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
一种新的三组分van Leusen策略已经被开发出来,用于从容易获得的丙胺、甲苯基甲基异氰化物和水中高效、直接地合成有价值的3,4-二取代吡咯。这种转化通过与异氰酸甲酯[3 + 2]环加成形成吡咯骨架和顺序亲核取代、开环和去质子化/质子化级联过程进行。该反应提供了一个方便的模块化途径,通过在一个步骤中从现成的无环前体形成三个新的键和一个杂环,得到具有芳基和苯甲酰基的吡咯衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
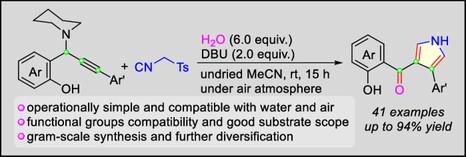
Modular Synthesis of 3,4-Disubstituted Pyrroles Through Three-Component Van Leusen Reaction of Propargylamines with Tosylmethyl Isocyanide and Water
A novel three-component van Leusen strategy has been developed for the efficient and straightforward synthesis of valuable 3,4-disubstituted pyrroles from easily available propargylamines, tosylmethyl isocyanide, and water. This transformation advances through the formation of a pyrrole framework via [3 + 2] cycloaddition with tosylmethyl isocyanide and a sequential nucleophilic substitution, ring-opening, and deprotonation/protonation cascade process. This reaction provides a convenient modular access to pyrrole derivatives with aryl and benzoyl groups through the formation of three new bonds and one heterocycle from readily available acyclic precursors in a single step.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


