Rh金属催化剂在NO-CO反应中的理论研究:活性和选择性的显著表面依赖性
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
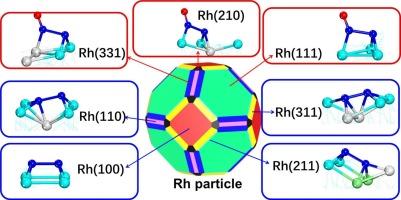
金属颗粒催化活性和选择性的表面依赖性对理解催化和设计新型催化剂具有重要意义。然而,正确的知识是有限的。本文采用DFT计算和微动力学模拟研究了Rh金属催化剂的NO-CO反应,以阐明活性和选择性的表面依赖性。反应性N-O债券乳沟减少Rh的顺序(100) 祝辞 Rh(110) ∼ Rh(211) ∼ Rh(311) 祝辞 Rh(210) 祝辞 Rh(331) ∼ Rh(111)。二氧化碳形成反应性降低的顺序Rh(100) 祝辞 Rh(331) 祝辞 Rh(110) ∼ Rh(111) 祝辞 Rh(311) ∼ Rh(211) ∼ Rh(210)。反应为一氧化二氮的形成降低的顺序Rh(100) ∼ Rh(210) ∼ Rh(331) 祝辞 Rh(110) 祝辞 Rh(111) 祝辞 Rh(211) 祝辞 Rh(311)。除了Rh(210)外,由于N2O解吸能小,N2O通过N2 - o键解理生成N2在几乎所有表面都不容易发生。反应性的N2通过重组形成两个吸附N原子(名为2 N重组)减少Rh的顺序(110) 祝辞 Rh(211) 祝辞 Rh(100) 祝辞 Rh(331) 祝辞 Rh(311) 祝辞 Rh(210) 祝辞 Rh(111)。利用这些计算结果进行的微动力学模拟表明,Rh(100)、Rh(110)、Rh(211)和Rh(311)表面在低温和高温下都对NO-CO反应有活性,但热力学最稳定的Rh(111)表面只在高温下有活性。具有Rh(100)、Rh(110)、Rh(211)和/或Rh(311)面的Rh金属颗粒被认为是优良的催化剂,而具有Rh(111)、Rh(210)和/或Rh(331)面的Rh金属颗粒则不是。考虑到各面暴露比,推荐具有Rh(100)和/或Rh(311)的Rh金属颗粒作为有前景的催化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Theoretical study on Rh metal catalyst in NO–CO reaction: Significant surface dependences of activity and selectivity
Surface-dependences of catalytic activity and selectivity of metal-particle are crucial for understanding catalysis and designing new catalysts. However, correct knowledge is limited. Herein, Rh metal catalyst for NO–CO reaction is investigated using DFT calculations with microkinetic simulation to elucidate surface-dependences of activity and selectivity. Reactivity for N‒O bond cleavage decreases in the order Rh(100) > Rh(110) ∼ Rh(211) ∼ Rh(311) > Rh(210) > Rh(331) ∼ Rh(111). Reactivity for CO2 formation decreases in the order Rh(100) > Rh(331) > Rh(110) ∼ Rh(111) > Rh(311) ∼ Rh(211) ∼ Rh(210). Reactivity for N2O formation decreases in the order Rh(100) ∼ Rh(210) ∼ Rh(331) > Rh(110) > Rh(111) > Rh (211) > Rh(311). N2 formation from N2O via N2‒O bond cleavage is not easy to occur in almost all surfaces due to small N2O desorption energy except Rh(210). Reactivity for N2 formation via recombination of two adsorbed N atoms (named 2 N recombination) decreases in the order Rh(110) > Rh(211) > Rh(100) > Rh(331) > Rh(311) > Rh(210) > Rh(111). Microkinetic simulation using these computational results indicates that Rh(100), Rh(110), Rh(211), and Rh(311) surfaces are active for NO–CO reaction at both low and high temperatures but the thermodynamically most stable Rh(111) surface is active only at high temperature. Rh metal particle with Rh(100), Rh(110), Rh(211), and/or Rh(311) facets is proposed as excellent catalyst, whereas Rh metal particle with Rh(111), Rh(210), and/or Rh(331) facets is not. Considering exposure ratios of facets, Rh metal particle with Rh(100) and/or Rh(311) is recommended as a promising catalyst.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


