强金属载体相互作用驱动的超小高熵合金纳米颗粒选择性加氢合成
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
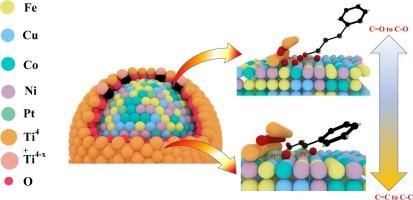
高熵合金(HEAs)由于具有几乎无限的活性位点而引起了人们的广泛关注。然而,由于其固有的热力学不稳定性,超小HEA纳米颗粒的合成仍然具有挑战性。在本研究中,我们证明了强金属-载体相互作用(SMSI)可以通过界面稳定有效地抑制团聚。通过利用基于淬火的方法,我们成功地合成了具有超低负载(<0.17 wt%)、超小粒径(<3.7 nm)和smsi诱导封装层的HEA催化剂。这种保护层的形成使得对催化剂的结构和电子性质的精确控制成为可能。为了考察肉桂醛在选择性加氢反应中的催化性能,以肉桂醛为模型α,β-不饱和醛。结果表明,HEA- cu8pt2中Pt比例越低,HCAL选择性越强,达到93.6 %,这是由于TiO2与HEA之间的界面电子环境发生了改变,促进了Pt2+的形成和强氢溢出。这项工作为通过SMSI效应设计和调控超小型HEA催化剂提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Strong metal support interaction driven synthesis of ultrasmall high entropy alloy nanoparticles for selective hydrogenation
High-entropy alloys (HEAs) have garnered significant attention due to their nearly infinite number of unprecedented active sites. However, the synthesis of ultrasmall HEA nanoparticles remains challenging due to their inherent thermodynamic instability. In this study, we demonstrate that a strong metal-support interaction (SMSI) can effectively suppress agglomeration through interfacial stabilization. By leveraging a quenching-based approach, we successfully synthesized HEA catalysts with ultralow loading (<0.17 wt%), ultrasmall particle sizes (<3.7 nm), and an SMSI-induced encapsulation layer. The formation of this protective layer enables precise control over the structural and electronic properties of catalyst. To probe the catalytic performance, cinnamaldehyde was employed as a model α,β-unsaturated aldehyde in selective hydrogenation reactions. The results reveal that a lower Pt ratio in HEA-Cu8Pt2 enhances HCAL selectivity, reaching 93.6 %, which is attributed to the modified interfacial electronic environment between TiO2 and HEA, promoting Pt2+ species formation and strong hydrogen spillover. This work provides new insights into the design and regulation of ultrasmall HEA catalysts through SMSI effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


