原料和功能化稻壳二氧化硅选择性去除铁和铝作为磷石膏浸出液的预处理步骤,随后回收稀土元素
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
酸浸法从磷石膏中回收稀土元素已被证明是有效的。然而,所得的渗滤液通常含有高浓度的干扰离子,如Fe3+和Al3+,这阻碍了随后的稀土回收步骤。本研究提出了一种新的磷石膏浸出液预处理方法,重点研究了利用原料稻壳衍生二氧化硅(Si)和3-氨基丙基三乙氧基硅烷(Si- apts)和2,6-吡啶二羧酸(Si- apts - co)功能化的稻壳衍生二氧化硅吸附去除Fe3+和Al3+。表征结果表明二氧化硅的制备和功能化是成功的。原料和功能化稻壳衍生二氧化硅对Fe3+和Al3+的吸附高度依赖于pH,在pH 5.0时Fe3+去除率为30 %,在pH 5.5时Al3+去除率为90 %。对Fe3+离子的吸附速度更快,拟二阶模型更符合两种金属离子的动力学数据。Freundlich模型很好地拟合了平衡数据。在333 K、吸附剂用量为1.5 g L−1时,Fe3+和Al3+的最高qe值分别为34.16 mg g−1(含Si-APTS- co)和32.42 mg g−1(含Si-APTS)。功能化改变了硅的热行为,硅表现为放热吸附,而功能化硅表现为吸热吸附。该吸附剂在去除磷石膏渗滤液中的Fe3+和Al3+方面表现出良好的潜力。此外,它们对REE3+离子没有亲和力,这意味着在提出的预处理步骤中没有这些元素的损失。本文章由计算机程序翻译,如有差异,请以英文原文为准。
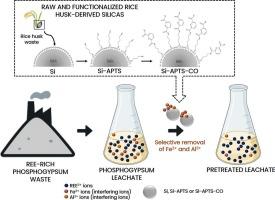
Selective removal of Fe and Al by raw and functionalized rice husk silicas as a pre-treatment step of phosphogypsum leachates for subsequent recovery of rare earth elements
Rare earth elements (REE) recovery from phosphogypsum by acid leaching has already proven effective. However, the obtained leachates often contain high concentrations of interfering ions, such as Fe3+ and Al3+, which hinder subsequent REE recovery steps. This work proposes a novel pre-treatment approach for phosphogypsum leachates, focusing on the removal of Fe3+ and Al3+ through adsorption using raw rice husk-derived silica (Si) and rice husk-derived silica functionalized with 3-aminopropyl triethoxysilane (Si-APTS) and 2,6-pyridinedicarboxylic acid (Si-APTS-CO). The characterization results demonstrated the success of the silica production and functionalization. The Fe3+ and Al3+ adsorption onto the raw and functionalized rice huks-derived silicas was highly pH-dependent, reaching 30 % Fe3+ removal at pH 5.0 and 90 % Al3+ removal at pH 5.5. The adsorption of Fe3+ ions was faster, and the pseudo-second-order model demonstrated greater suitability to the kinetic data of both metal ions. The Freundlich model fitted the equilibrium data well. The highest qe values for Fe3+ and Al3+ were, respectively, 34.16 mg g−1 (with Si-APTS-CO) and 32.42 mg g−1 (with Si-APTS) at 333 K and an adsorbent dosage of 1.5 g L−1. Functionalization changed the thermal behavior, with Si showing exothermic adsorption, while the functionalized silicas exhibited endothermic adsorption. The adsorbents demonstrated promising potential to remove Fe3+ and Al3+ from a real-world phosphogypsum leachate. In addition, they did not show affinity for the REE3+ ions, meaning that there are no losses of these elements in the proposed pre-treatment step.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


