线粒体蛋白酶ClpP缺乏可预防糖尿病肾病的小管间质损伤。
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
线粒体质量控制(MQC)失衡与糖尿病肾病(DKD)的小管间质损伤有关。线粒体未折叠蛋白反应(UPRmt)是MQC所需的应激适应性转录反应。酪蛋白溶解肽酶P (ClpP)是UPRmt蛋白水解系统的关键组成部分,在调节线粒体功能中起着重要作用,其有益和有害的结果都有。然而,它对肾脏病理生物学的影响仍不清楚。我们观察到ClpP分布在肾小管中,在DKD患者和db/db小鼠的肾脏中ClpP显著升高,并伴有upmt相关分子伴侣热休克蛋白60 (HSP60)、热休克蛋白10 (HSP10)和激活转录因子5 (ATF5)的表达升高,与肾脏氧化应激、细胞凋亡和肾小管间质纤维化呈正相关。ClpP shRNA可减轻糖尿病小鼠小管细胞凋亡、氧化损伤和小管间质损伤。HSP60、HSP10和ATF5的表达被抑制,表明ClpP的降低抑制了UPRmt的激活。在体外,ClpP定位于HK-2细胞的线粒体中。高糖(HG)处理上调了ClpP表达和uprmt相关蛋白,同时增强了线粒体活性氧(mtROS)、纤维化标志物和细胞凋亡。这些改变被ClpP siRNA减少。相反,ClpP过表达进一步加剧了HK-2细胞中的这些异常,而这些促进作用被UPRmt抑制部分逆转。我们的研究结果表明,ClpP缺乏通过抑制过度的UPRmt激活来改善DKD的肾氧化应激和小管间质损伤。这些结果表明ClpP是一个有价值的治疗DKD的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
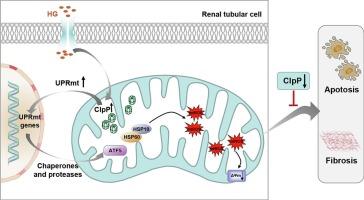
Mitochondrial protease ClpP deficiency protects against tubulointerstitial damage in diabetic kidney disease
Mitochondrial quality control (MQC) imbalance has been implicated in tubulointerstitial damage of diabetic kidney disease (DKD). The mitochondrial unfolded protein response (UPRmt) is a stress-adaptive transcriptional response required for MQC. Caseinolytic peptidase P (ClpP), the critical component of the UPRmt proteolytic system, plays an essential role in regulating mitochondrial function with both beneficial and detrimental outcomes. Still, its effects on kidney pathobiology remain unclear. Here, we observed that ClpP was distributed in renal tubules and was significantly increased in the kidneys of DKD patients and db/db mice, accompanied by increased expression of the UPRmt-related molecular chaperones heat shock protein 60 (HSP60), heat shock protein 10 (HSP10) and activating transcription factor 5 (ATF5) and positively correlated with renal oxidative stress, cell apoptosis and tubulointerstitial fibrosis. ClpP shRNA alleviated tubular cell apoptosis, oxidative damage and tubulointerstitial injury in diabetic mice. The expression of HSP60, HSP10 and ATF5 was inhibited, indicating that lowering ClpP suppressed UPRmt activation. In vitro, ClpP was localized in the mitochondria of HK-2 cells. High glucose (HG) treatment upregulated ClpP expression and UPRmt-related proteins, concurrent with enhanced mitochondrial reactive oxygen species (mtROS), fibrosis markers and apoptosis. These alterations were reduced by ClpP siRNA. Instead, ClpP overexpression further exacerbated these abnormalities in HK-2 cells, while these facilitation effects were partially reversed by UPRmt suppression. Our results indicated that ClpP deficiency ameliorated renal oxidative stress and tubulointerstitial injury in DKD by inhibiting excessive UPRmt activation. These results suggest that ClpP is a valuable therapeutic target for DKD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


