白丹素通过激活KLF2和抑制ZWINT/NF-κB,重塑舌鳞癌肿瘤免疫微环境,克服顺铂耐药。
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
舌鳞状细胞癌(TSCC)是一个治疗挑战,由于常见的顺铂耐药和免疫抑制肿瘤免疫微环境(TIME)。有效的战略必须解决这两个障碍。我们在顺铂敏感和耐药的TSCC细胞以及异种移植、患者来源的异种移植和人源化小鼠模型中评估了白丹素(PLB)的抗肿瘤作用。PLB下调ZWINT,抑制NF-κB信号,从而恢复耐药细胞对顺铂的敏感性。在体内,PLB显著抑制肿瘤生长,增强顺铂疗效。在机制上,PLB稳定了KLF2,促进JAK1/STAT2信号,TIME重塑和CD8+ t细胞功能的部分恢复。这些双重作用——通过ZWINT/NF-κB抑制的肿瘤化学致敏和通过KLF2激活的免疫重编程——在功能上相互关联,因为plb启动的T细胞释放的细胞因子增强了肿瘤细胞中NF-κB的抑制。总的来说,我们的研究结果确定了PLB是一种低成本的小分子,将顺铂致敏与免疫激活结合在一起,为顺铂难治性TSCC提供了一种有希望的辅助策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
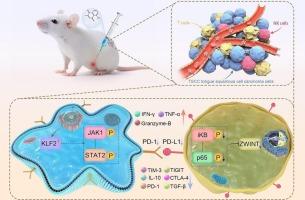
Plumbagin remodels the tumor immune microenvironment to overcome cisplatin resistance in tongue squamous cell carcinoma via KLF2 activation and ZWINT/NF-κB inhibition
Tongue squamous cell carcinoma (TSCC) is a therapeutic challenge due to frequent cisplatin resistance and an immunosuppressive tumor immune microenvironment (TIME). Effective strategies must address both barriers. We evaluated the antitumor effects of plumbagin (PLB) in cisplatin-sensitive and -resistant TSCC cells, as well as in xenograft, patient-derived xenograft, and humanized mouse models. PLB downregulated ZWINT and inhibited NF-κB signaling, thereby restoring cisplatin sensitivity in resistant cells. In vivo, PLB significantly suppressed tumor growth and enhanced cisplatin efficacy. Mechanistically, PLB stabilized KLF2, promoting JAK1/STAT2 signaling, TIME remodeling, and partial restoration of CD8+ T-cell function. These dual actions—tumor chemosensitization via ZWINT/NF-κB inhibition and immune reprogramming via KLF2 activation—were functionally interconnected, as cytokines released by PLB-primed T cells reinforced NF-κB suppression in tumor cells. Collectively, our findings identify PLB as a low-cost small molecule that integrates cisplatin sensitization with immune activation, offering a promising adjunctive strategy for cisplatin-refractory TSCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


