钙调磷酸酶抑制在儿童类固醇抵抗性肾病综合征中的疗效
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
本研究旨在为钙调磷酸酶抑制剂(CNI)治疗儿童类固醇抵抗性肾病综合征(SRNS)的疗效提供证据。方法278例接受一线CNI治疗的SRNS患儿,使用竞争风险分析评估累积缓解和肾衰竭发生率。Kaplan-Meier和Cox回归分析分析肾脏生存,确定CNI反应性的预测因素,并估计在CNI治疗或停止CNI治疗时突破性蛋白尿发作的累积发生率。使用多变量线性混合效应模型评估CNI剂量和谷底水平对蛋白尿的影响。结果在给予CNI 6个月内,219例非遗传性SRNS患者的蛋白尿减少了84%(四分位数范围:80%-87%),59例遗传性SRNS患者的蛋白尿减少了58%(42%-70%),但后者在9至12个月内恢复到治疗前水平。219例非遗传性SRNS中有91例(42%)和59例遗传性SRNS中有6例(10%)完全缓解,但只有53例(24%)和2例(3%)非遗传性SRNS持续缓解。蛋白尿减少,但没有达到完全缓解,与使用较高的CNI剂量有关。在非遗传性SRNS中,12个月和24个月后,CNI治疗发生突破性蛋白尿的累积风险分别为51%(40%-62%)和65%(54%-75%)。完全缓解患者停药后12个月和24个月的复发风险分别为40%(22%-59%)和50%(30%-69%)。非遗传性SRNS患儿的肾脏存活率优于cni应答患儿(92% vs. 15岁时的42%),与突破性蛋白尿事件无关。结论我们的研究提供了关于CNI治疗在非遗传性和遗传性SRNS中的程度、动态、剂量-反应关系和长期功能影响的真实证据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
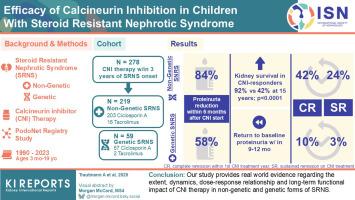
Efficacy of Calcineurin Inhibition in Children With Steroid-Resistant Nephrotic Syndrome
Introduction
We aimed to provide evidence for the efficacy of calcineurin inhibitor (CNI) treatment in children with steroid-resistant nephrotic syndrome (SRNS).
Methods
In 278 SRNS children receiving first-line CNI treatment, cumulative remission and kidney failure incidence were estimated using competing risk analysis. Kaplan-Meier and Cox regression analyses were performed to analyze kidney survival, identify predictors of CNI responsiveness and estimate the cumulative incidence of breakthrough proteinuria episodes on or off CNI treatment. The impact of CNI dosage and trough levels on proteinuria was assessed using multivariable linear-mixed effects modeling.
Results
Within 6 months of CNI administration, proteinuria was reduced by 84% (interquartile range: 80%–87%) in 219 nongenetic SRNS cases and by 58% (42%–70%) in 59 genetic SRNS cases but returned to pretreatment level in the latter group within 9 to 12 months. Whereas complete remission was observed in 91 of 219 nongenetic SRNS cases (42%) and 6 of 59 genetic SRNS cases (10%), remission was sustained in 53 nongenetic (24%) and 2 genetic (3%) cases only. Proteinuria reduction, but not attainment of complete remission, was associated with the use of higher CNI doses. The cumulative risk of breakthrough proteinuria on CNI treatment was 51% (40%–62%) and 65% (54%–75%) after 12 and 24 months, respectively, in nongenetic SRNS. The postdiscontinuation relapse risk in patients with complete remission was 40% (22%–59%) and 50% (30%–69%) after 12 and 24 months, respectively. Kidney survival in nongenetic SRNS was superior in CNI-responsive children (92% vs. 42% at 15 years), independent of breakthrough proteinuria episodes.
Conclusion
Our study provides real-world evidence regarding the extent, dynamics, dose-response relationship, and long-term functional impact of CNI therapy in nongenetic and genetic forms of SRNS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


