B细胞胞外囊泡影响黑色素瘤对免疫检查点治疗的反应
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
肿瘤免疫微环境是一个动态的生态系统,其中B细胞在调节免疫检查点阻断(ICB)治疗反应中发挥关键作用。虽然传统上被认为是肿瘤免疫的被动参与者,但最近的证据表明,B细胞积极影响抗肿瘤反应。本研究探讨了B细胞及其细胞外囊泡(EVs)在黑色素瘤对ICB的反应中的作用。回顾性荟萃分析显示,在ICB应答者的预处理中,B细胞富集增加。功能分析显示B细胞耗竭损害T细胞介导的肿瘤细胞毒性。我们分析了来自黑色素瘤的EVs,发现CD19 + EVs中的miR-99a-5p在应答者中上调。在B细胞中沉默miR-99a-5p可降低T细胞抗肿瘤活性,提示其在免疫调节中的作用。在机制上,miR-99a-5p通过类开关重组促进B细胞成熟。这些发现强调了B细胞对黑色素瘤免疫治疗的影响,为针对B细胞相关途径的新治疗策略提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
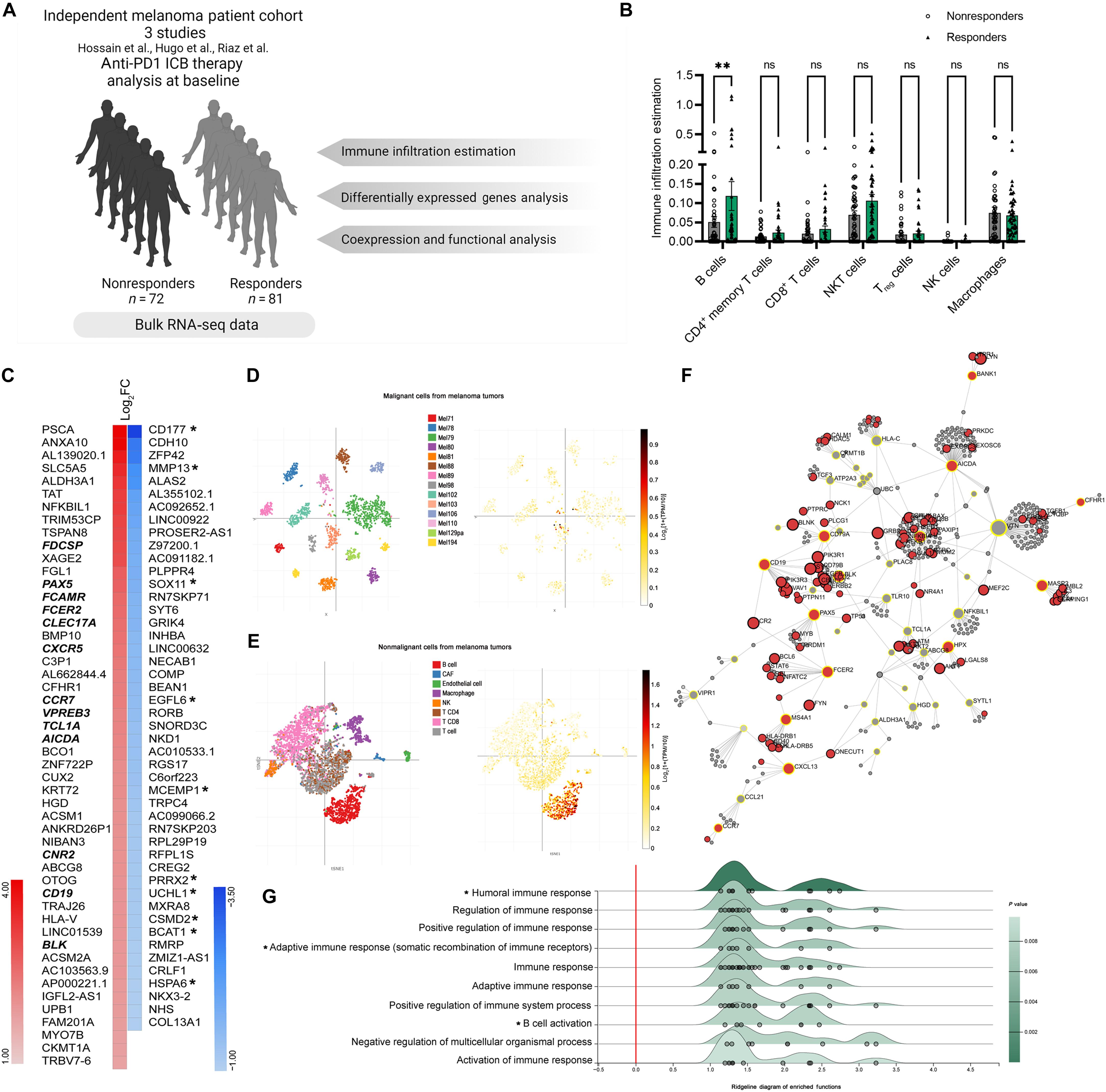
B cell extracellular vesicles influence melanoma response to immune checkpoint therapy
The immune tumor microenvironment is a dynamic ecosystem where B cells play critical roles in modulating immune checkpoint blockade (ICB) therapy responses. While traditionally seen as passive players in tumor immunity, recent evidence suggests that B cells actively influence antitumor responses. This study examines the role of B cells and their extracellular vesicles (EVs) in melanoma responses to ICB. Retrospective meta-analyses reveal increased B cell enrichment in ICB responders’ pretreatment. Functional assays show that B cell depletion impairs T cell–mediated tumor cytotoxicity. EVs from melanoma tumors were analyzed, identifying miR-99a-5p in CD19+ EVs as up-regulated in responders. Silencing miR-99a-5p in B cells reduces T cell antitumor activity, suggesting its role in immune modulation. Mechanistically, miR-99a-5p promotes B cell maturation via class-switch recombination. These findings underscore B cells’ impact on melanoma immunotherapy, offering insights into novel therapeutic strategies targeting B cell–related pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


