噬菌体裂解蛋白Lys M作为楔子阻断MurJ构象变化
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
许多抗生素针对基本的细胞过程。为了对抗多重耐药细菌,需要新的抗菌策略。在大肠杆菌的肽聚糖生物生成途径中,脂质II翻转酶MurJ是一种必需的膜蛋白。来自列文病毒噬菌体的37个残基蛋白M,被称为Lys M或Sgl M,靶向MurJ并诱导细胞裂解;然而,其分子机制尚不清楚。在这里,我们展示了MurJ/Lys M (JM)配合物在3.09埃分辨率下的低温电镜结构,揭示了Lys M与MurJ的TM2和TM7之间的裂缝相互作用,将MurJ锁定在一个向外的构象中,而Lys M就像一个楔子。丙氨酸扫描诱变和拉下实验揭示了Lys M功能的关键残基,分子动力学模拟表明Lys M稳定了MurJ的外向状态。这些发现证明了一种前所未有的噬菌体衍生的阻断脂质II转运的机制,为设计靶向murj的抗菌药物提供了结构框架。本文章由计算机程序翻译,如有差异,请以英文原文为准。
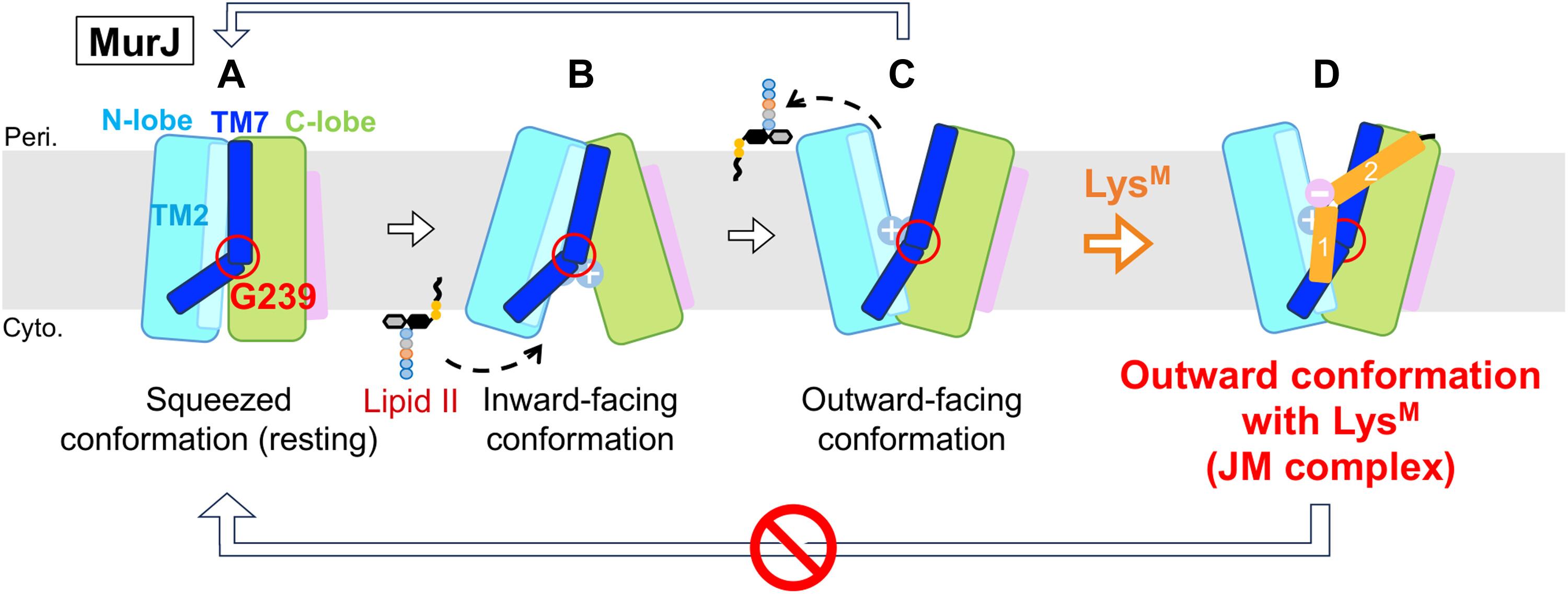
Phage lysis protein LysM acts as a wedge to block MurJ conformational changes
Many antibiotics target essential cellular processes. To combat multidrug-resistant bacteria, new antibacterial strategies are needed. In the peptidoglycan biogenesis pathway in Escherichia coli, MurJ, the lipid II flippase, is an essential membrane protein. The 37-residue protein M from the Levivirus phage, known as LysM or SglM, targets MurJ and induces cell lysis; however, its molecular mechanism remains unclear. Here, we present the cryo-EM structure of the MurJ/LysM (JM) complex at 3.09-angstrom resolution, revealing that LysM interacts with the crevasse between TM2 and TM7 of MurJ, locking MurJ in an outward-facing conformation, with LysM acting like a wedge. Alanine-scanning mutagenesis and pull-down assays revealed key residues responsible for LysM function, and molecular dynamics simulations showed that LysM stabilizes MurJ’s outward-facing state. These findings demonstrate an unprecedented phage-derived mechanism for blocking lipid II transport, providing a structural framework for designing MurJ-targeted antimicrobial agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


