用于无排放直接液体燃料电池的氧化还原电位工程杂多酸再生燃料
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
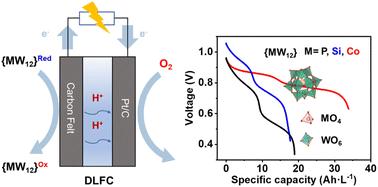
为了解决传统直接液体燃料电池(dlfc)氧化动力学迟缓、贵金属依赖和碳排放等问题,本研究提出了一种利用keggin型杂多酸(hpa)作为可回收燃料的新策略。通过对磷钨酸({PW12})、硅钨酸({SiW12})和钴钨酸({CoW12})电化学性能的系统比较,结合密度泛函理论(DFT)计算,阐明了中心原子价态和质子-电子耦合效应对氧化还原电位分布的调控机制。结果表明,由于低价Co(II)中心和质子耦合的电子转移机制,{CoW12}表现出明显集中的四电子还原电位范围(- 0.035 ~ - 0.151 V vs. SHE),有效抑制了析氢副反应,使电子充分利用。实验验证表明,在环境温度和非加湿条件下以及阳极无贵金属操作下,基于{CoW12}的DLFC在0.58 V下的能量密度为27.2 Wh L−1,峰值功率密度为0.529 W cm−2,分别比报道的{PW12}系统提高了118%和46%。该系统在30小时内也表现出稳定的放电性能。本研究为设计高容量、低成本的杂多酸基燃料电池系统提供了理论基础和材料创新范例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Redox potential-engineered heteropolyacid regenerative fuels for emission-free direct liquid fuel cells
To address the sluggish oxidation kinetics, noble metal dependency, and carbon emissions of traditional direct liquid fuel cells (DLFCs), this study proposes a novel strategy utilizing Keggin-type heteropolyacids (HPAs) as recyclable fuels. Through a systematic comparison of the electrochemical properties of phosphotungstic acid ({PW12}), silicotungstic acid ({SiW12}), and cobaltotungstic acid ({CoW12}), combined with density functional theory (DFT) calculations, we elucidate the regulatory mechanism of central atom valence states and proton–electron coupling effects on redox potential distribution. The results reveal that {CoW12}, owing to the low-valent Co(II) center and proton-coupled electron transfer mechanism, exhibits a significantly concentrated four-electron reduction potential range (−0.035 to −0.151 V vs. SHE), effectively suppressing hydrogen evolution side reactions and enabling full electron utilization. Experimental validation demonstrates that the {CoW12}-based DLFC achieves an energy density of 27.2 Wh L−1 and a peak power density of 0.529 W cm−2 at 0.58 V at ambient temperature and under non-humidified conditions and anode noble metal-free operation, representing 118% and 46% improvements over the reported {PW12} system, respectively. The system also demonstrates stable discharge performance over 30 hours. This work provides a theoretical foundation and material innovation paradigm for designing high-capacity, low-cost heteropolyacid-based fuel cell systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


