单给体环丙烷的有机光氧化还原和钴催化1,2-二官能化
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
单给体环丙烷中未激活的C─C和C─H键的选择性功能化是有机合成中一个重要但具有挑战性的目标。尽管大量的研究报告已经记录了这些底物的1,3-二功能化,但迄今为止1,2-二功能化的有效策略尚未被证明是难以捉摸的。在此,我们报告了一种新的催化策略,使单给体环丙烷的1,2-双官能化。该方法通过有机光氧化还原和钴催化体系的融合,实现了选择性开环和均酶氧化,通过单电子转移、亲核加成和无受体脱氢的双重顺序实现。这一前所未有的工艺颠覆了单给体环丙烷功能化中主要的1,3选择性,同时建立了以可持续方式锻造β-芳基-α′-氧酮衍生物的流线平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
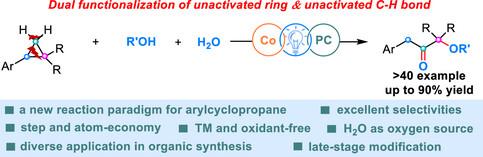
Organophotoredox and Cobalt-catalyzed 1,2-Difunctionalization of Mono-Donor Cyclopropanes
Selective functionalization of unactivated C─C and C─H bonds in mono-donor cyclopropanes represents a significant yet challenging goal in organic synthesis. Although numerous reported studies have documented the 1,3-difunctionalization of these substrates, effective strategies for 1,2-difunctionalization have so far proven elusive. Herein, we report a novel catalytic strategy enabling the 1,2-difunctionalization of mono-donor cyclopropanes. By merging of organophotoredox and cobalt catalytic system, this method achieves selective ring-opening and homobenzylic oxygenation, facilitated by a two-fold sequence involving single electron transfer, nucleophilic addition, and acceptorless dehydrogenation. This unprecedented process overturns the predominant 1,3-selectivity in mono-donor cyclopropane functionalization, concurrently establishing a streamlined platform for forging β-aryl-α′-oxyketone derivatives in a sustainable manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


