生物启发多孔离子超交联聚合物的敏捷构建增强CO2捕获和转化
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
脱氧核糖核酸(DNA)中生物碱之间的氢键相互作用维持了双螺旋结构的稳定性。受这一原理的启发,在本工作中,我们提出了一种通过生物碱和二氯联苯(BP)同时季铵盐化和Friedel-Crafts烷基化反应一锅合成离子超交联聚合物(iHCPs)的方法。这些iHCPs具有氢键能力,丰富的碱性和离子活性位点,以及具有超高表面积(高达1480 m2·g-1)的分层多孔结构。优化后的iHCPs AN-BP(1:3)在273 K和1 bar条件下具有154.4 mg·g-1的CO2吸附性,是一种可回收的催化剂,无需额外的助催化剂即可将CO2和环氧化物无溶剂转化为环状碳酸盐。原位FTIR光谱和密度泛函理论(DFT)计算表明,碱性基团增强了CO2吸附,并促进氯离子通过氢键促进环氧化物开环反应,从而降低了相对吉布斯自由能垒。这项工作通过将孔隙工程与氢键催化相结合,建立了离子聚合物的生物灵感设计范例,从而促进了二氧化碳的捕获和转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
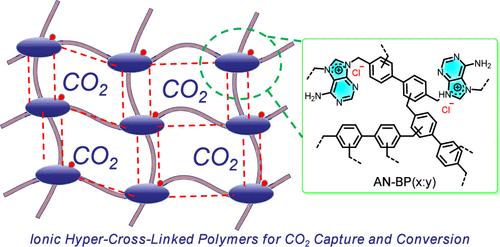
Agile Construction of Bioinspired Porous Ionic Hyper-Crosslinked Polymers for Enhanced CO2 Capture and Conversion
Hydrogen bond interactions between alkaloids in deoxyribonucleic acid (DNA) maintain the stability of the double helix structure. Inspired by this principle, in this work, we present a one-pot synthesis of ionic hyper-cross-linked polymers (iHCPs) via simultaneous quaternization and Friedel–Crafts alkylation reactions of alkaloids and biphenyl dichloride (BP). These iHCPs exhibited hydrogen bonding capabilities, abundant alkaline and ionic active sites, as well as hierarchically porous structures with ultrahigh surface areas (up to 1480 m2·g–1). The optimized iHCPs, AN-BP(1:3), demonstrated exceptional CO2 adsorption (154.4 mg·g–1 at 273 K and 1 bar) and served as a recyclable catalyst for the solvent-free conversion of CO2 and epoxides into cyclic carbonates without requiring additional cocatalysts. In situ FTIR spectroscopy and density functional theory (DFT) calculations revealed that basic groups enhanced the CO2 adsorption and facilitated chloride ions in promoting the epoxide ring-opening reaction via hydrogen bonding, thereby reducing the relative Gibbs free energy barrier. This work establishes a bioinspired design paradigm for ionic polymers by integrating pore engineering with hydrogen-bond catalysis, thus advancing both CO2 capture and conversion.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


