负载cu的碳催化剂对过硫酸氢盐的高效活化降解高浓度刚果红
IF 6.2
1区 农林科学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
摘要
以叶绿素钠铜盐为原料,采用一步热解法合成了Cu簇载碳催化剂。cuc700活化过硫酸氢盐(PDS)对高浓度刚果红(100 mg/L)的降解效率为90.69 %(10 min)。动态降解600 min后,刚果红的降解率为90.26 %。提高热处理温度可以提高铜团簇的结晶度。热处理后块状低孔CuC催化剂中吡咯氮含量增加,生成Cu+种。中性环境最有利于刚果红的降解;然而,腐植酸的存在抑制了降解速率。在刚果红的降解过程中,主要由1O2和•O2−等物质介导,第一线原理计算表明,碳层上负载的Cu团簇显著提高了PDS的吸附能,导致O-O键的长度从1.469增加到1.512 Å。碳上的氮掺杂和Cu簇负载改善了CuC700对PDS的吸附和电子传递。刚果红分子内的N-N键作为主要裂解位点,定量构效关系分析表明,所产生的降解中间体的集体毒性降低。最后,提出了刚果红的潜在降解途径和机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
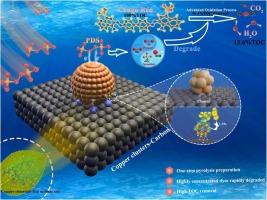
Efficient activation of peroxodisulfate by Cu-loaded carbon catalysts for the degradation of highly concentrated Congo red
Cu cluster-loaded carbon (CuC) catalysts were synthesized through a one-step pyrolysis process utilizing chlorophyllin sodium copper salt. The degradation efficiency of CuC700-activated peroxodisulfate (PDS) against high concentration Congo red (100 mg/L) was 90.69 % after 10 min. The degradation rates of Congo red after 600 min of dynamic degradation were 90.26 %. Increasing the heat treatment temperature enhances the crystallinity of Cu clusters. After heat treatment, the pyrrole nitrogen content in the blocky, low-porosity CuC catalyst increases, and Cu+ species are generated. A neutral environment is most favorable for the degradation of Congo red; however, the presence of humic acid inhibits the degradation rate. During the Congo red degradation, it is primarily mediated by species such as 1O2 and •O2−, with first-principles calculations indicating that Cu clusters loaded on carbon layers markedly enhance the adsorption energy of PDS, leading to an increase in the length of the O-O bond from 1.469 to 1.512 Å. Nitrogen doping and Cu cluster loading on carbon improve the adsorption and electron transport from CuC700 to PDS. The N-N bond within the Congo red molecule as primary cleavage site, and quantitative structure-activity relationship analyses indicate a reduction in the collective toxicity of the resulting degradation intermediates. Finally, potential degradation pathways and mechanisms for Congo red are proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial Crops and Products
农林科学-农业工程
CiteScore
9.50
自引率
8.50%
发文量
1518
审稿时长
43 days
期刊介绍:
Industrial Crops and Products is an International Journal publishing academic and industrial research on industrial (defined as non-food/non-feed) crops and products. Papers concern both crop-oriented and bio-based materials from crops-oriented research, and should be of interest to an international audience, hypothesis driven, and where comparisons are made statistics performed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


