镍包埋共价有机框架双光催化析氢和交叉偶联催化
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
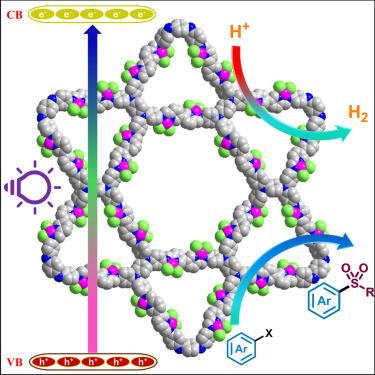
本文报道了以[3,3 ' -联吡啶]-6,6 ' -二甲醛(3,3 ' -BiPy)为电子受体,以四akis(4-氨基苯基)-1,4-苯二胺(TPDA)为供体,设计和合成了一种新型亚胺连接的二维共价有机骨架(COF) TPDA-BiPy-COF。COF的特点是吡啶-亚胺键,即Nimine-Ni-Nbipyridine,具有活性氮位,有利于质子还原为氢。为了提高光催化出氢性能,通过合成后金属化引入Ni(II)中心,形成TPDA-BiPy@NiX₂COF (X = Cl, Br)。镍(II)与亚胺和联吡啶氮原子的配位增强了框架的平面性和共轭性,从而提高了光催化活性。值得注意的是,TPDA-BiPy@Ni(II) COF在不需要助催化剂的情况下,在可见光下达到了34.13 mmol g⁻¹h的极好的出氢速率。此外,TPDA-BiPy@Ni(II)的金属氧化还原活性显示出其光催化C-S交叉偶联反应的前景。这种双功能催化剂突出了将镍纳入COFs的优势,为光催化和合成转化提供了一种具有成本效益和可持续性的贵金属基系统替代品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nickel-embedded covalent organic frameworks for dual photocatalytic hydrogen evolution and cross-coupling catalysis
This work reports the design and synthesis of a novel imine-linked 2D covalent organic framework (COF), TPDA-BiPy-COF, constructed from [3,3′-bipyridine]-6,6′-dicarboxaldehyde (3,3′-BiPy) as an electron acceptor and tetrakis(4-aminophenyl)-1,4-phenylenediamine (TPDA) as a donor. The COF features pyridyl-imine linkages, i.e., Nimine-Ni-Nbipyridine, with active nitrogen sites that facilitate proton reduction to hydrogen. To improve photocatalytic hydrogen evolution performance, Ni(II) centers were introduced via post-synthetic metalation, forming TPDA-BiPy@NiX₂ COF (X = Cl, Br). The coordination of Ni(II) with the imine and bipyridine nitrogen atoms enhanced framework planarity and conjugation, thereby boosting photocatalytic activity. Notably, TPDA-BiPy@Ni(II) COF achieved an excellent hydrogen evolution rate of 34.13 mmol g⁻¹ h⁻¹ under visible light, without requiring a cocatalyst. Furthermore, the metallaphotoredox activity of TPDA-BiPy@Ni(II) displayed its promise for photocatalyzed C–S cross-coupling reaction. This dual-functional catalyst highlights the advantage of incorporating nickel into COFs, offering a cost-effective and sustainable alternative to noble-metal-based systems for photocatalysis and synthetic transformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


