在低结合3d打印生物芯片中原位形成和培养细胞球体
IF 5.4
2区 工程技术
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
器官芯片和微流控系统为克服传统体外模型在临床前研究中的局限性提供了新的途径。然而,缺乏标准化和重要的测试药物与通常由聚二甲基硅氧烷(PDMS)制成的设备的非特异性结合仍然减缓了它们完全融入工业研究管道的速度。本研究的目标是使用全氟聚醚(PFPE)开发一种具有低结合特性的标准化3d打印生物芯片,允许长时间动态培养原位形成的细胞球体。我们首先记录了与制药公司相关的分子的非特异性结合以及PFPE与PDMS相比的机械和表面特性。然后设计了一种新的微结构生物芯片,并在PFPE上进行了3d打印,该芯片具有400 μL的腔室,包含384个微孔。3d打印制造方案已经详细考虑了关键参数,如紫外线曝光时间或后固化。最后,在动态条件下,每个芯片形成384个HepG2/C3a球体,并维持11天。在这些打印的PFPE生物芯片中培养的球体具有高活力、功能性和极化性,表明这种新的微生理系统可以替代PDMS设备。本文章由计算机程序翻译,如有差异,请以英文原文为准。
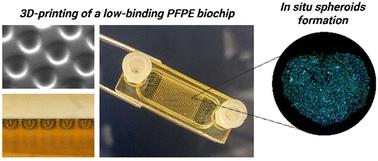
In situ formation and culture of cell spheroids in a low-binding 3D-printed biochip
Organ-on-a-chip and microfluidic systems offer new ways to overcome limitations from traditional in vitro models in preclinical studies. However, the lack of standardization and important non-specific binding of tested drugs to devices commonly made of polydimethylsiloxane (PDMS) still slow down their full integration into industrial research pipelines. The goal of this study is to develop a standardized 3D-printed biochip with low-binding properties using perfluoropolyether (PFPE), allowing long-time dynamic cultures of in situ formed cellular spheroids. We first documented the non-specific binding of molecules relevant for pharmaceutical companies and mechanical and surface properties of PFPE as compared with PDMS. A new microstructured biochip was then designed and 3D-printed in PFPE to offer a 400 μL chamber containing 384 microwells. The 3D-printing fabrication protocol has been detailed considering key parameters such as UV exposure time or postcuring. Finally, 384 HepG2/C3a spheroids were formed per chip under dynamic conditions and maintained for 11 days. The high viability, functionality and polarization of the spheroids cultured in these printed PFPE biochips showed the relevance of this new microphysiological system as an alternative to PDMS devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Lab on a Chip
工程技术-化学综合
CiteScore
11.10
自引率
8.20%
发文量
434
审稿时长
2.6 months
期刊介绍:
Lab on a Chip is the premiere journal that publishes cutting-edge research in the field of miniaturization. By their very nature, microfluidic/nanofluidic/miniaturized systems are at the intersection of disciplines, spanning fundamental research to high-end application, which is reflected by the broad readership of the journal. Lab on a Chip publishes two types of papers on original research: full-length research papers and communications. Papers should demonstrate innovations, which can come from technical advancements or applications addressing pressing needs in globally important areas. The journal also publishes Comments, Reviews, and Perspectives.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


