基于纳米颗粒的快速检测人血清戊型肝炎病毒生物传感器的研制与临床评价
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
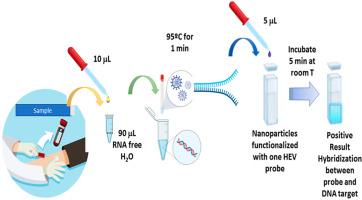
戊型肝炎病毒(HEV)基因3型是一种人畜共患病原体,在工业化国家日益得到认可,通常与食源性或动物接触传播有关。虽然大多数感染是自限性的,但疫情对公共卫生构成重大挑战,特别是在免疫功能低下人群和献血者中。早期病毒检测对于有效的疾病管理和遏制至关重要,因为它有助于确定感染聚集并实施公共卫生措施,以控制疾病的传播,特别是在社区环境中,这突出表明需要创新的快速吞吐量诊断平台。在这项研究中,我们提出了一种新型基于纳米粒子的遗传生物传感器的开发和全面的分析/临床验证,可以直接从人类血清中快速检测HEV,周转时间约为5分钟。该系统由三个独立的试剂盒组成,每个试剂盒都含有经过验证的核酸探针功能化的纳米颗粒,靶向保守的HEV基因组区域。当对临床HEV-3阳性样本进行检测时,该生物传感器与RT-qPCR完全一致,检测到的病毒载量低至1 IU/mL。此外,当对其他已知的人类病毒,即甲型肝炎、乙型肝炎、巨细胞病毒和爱泼斯坦-巴尔病毒进行测试时,它是一个高度特异性的系统。该平台便于携带,成本效益高,不需要核酸提取或扩增步骤,非常适合现场设置或即时检测(PoCT)。这种快速和可获得的方法有望扩大诊断能力,特别是在资源有限或实地环境中,它可以对早期发现疫情、快速诊断感染、跟踪疾病传播以及评估食品和水安全产生重大影响。发展快速的现场检测对于能够迅速作出公共卫生反应,从而支持全球消除病毒性肝炎的努力至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Development and Clinical Evaluation of a Nanoparticle-based Biosensor for Rapid Detection of Hepatitis E Virus in Human Serum
Hepatitis E virus (HEV) genotype 3 is a zoonotic pathogen increasingly recognized in industrialized countries that is often linked to foodborne or animal contact transmission. While most infections are self-limited, outbreaks pose a significant public health challenge, especially in immunocompromised populations and blood donation recipients. Early-stage viral detection is critical for effective disease management and containment, since it helps to identify clusters of infection and implement public health measures to control the spread of the disease, especially in community settings, which underscores the need for innovative rapid through-put diagnostic platforms. In this study, we present the development and comprehensive analytical/clinical validation of a novel nanoparticle-based genetic biosensor, enabling rapid HEV detection directly from human serum with a turnaround time of approximately five minutes. The system consists of three individual kits, each containing nanoparticles functionalized with validated nucleic acid probes targeting conserved HEV genomic regions. When tested on clinical samples positive for HEV-3, the biosensor showed full concordance with RT-qPCR, detecting viral loads as low as <1 IU/mL. Moreover, it is a highly specific system when tested against other known human virus, namely hepatitis A, hepatitis B, cytomegalovirus and, Epstein-Barr virus. The platform is portable, cost-effective, and does not require nucleic acid extraction or amplification steps, making it highly suitable for field settings or Point-of-Care Testing (PoCT). This rapid and accessible approach holds promise for expanding diagnostic capabilities, particularly in resource-limited or field settings, where it can have a great impact on early outbreak detection, rapid diagnosis of infections, tracking disease spread, and assessing food and water safety. The development of rapid, in field tests are crucial for enabling prompt public health responses, and thus, support the global effort to eliminate viral hepatitis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


