四取代烯烃不对称环氧化装置三氟甲基化季碳立体中心
IF 4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
四取代烯烃环氧化构造邻季碳中心一直是不对称合成中的一个难题。本文报道了通过三氟甲基酮与丙二腈的Knoevenagel缩合制备无环四取代烯烃的第一个催化对映选择性环氧化反应。(S)‐和(R)‐配置的环氧化物通常具有优异的收率(高达98%)和良好到高的对映选择性(高达89% ee),采用市售体系,包括竹本的催化剂和过氧化叔丁基(TBHP)。高活性的新型四取代烯烃允许在氧化条件下首次成功使用武本的催化剂。有趣的是,密度泛函理论研究表明,TBHP仅被氨基硫脲激活,卤素键相互作用在影响环氧化的立体化学结果中起着关键作用。衍生化提供了获得密集功能化的三氟甲基化化合物的途径,具有一个或两个相邻的碳季元立体中心,第二个中心以高度立体选择性的方式引入。本文章由计算机程序翻译,如有差异,请以英文原文为准。
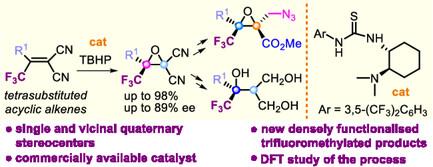
Installation of Trifluoromethylated Quaternary Carbon Stereocenters via Asymmetric Epoxidation of Tetrasubstituted Alkenes
The construction of vicinal quaternary carbon centers via epoxidation of tetrasubstituted alkenes remains a formidable challenge in asymmetric synthesis. Herein, a first catalytic enantioselective epoxidation of acyclic tetrasubstituted alkenes is reported, prepared through the Knoevenagel condensation of trifluoromethyl ketones with malononitrile. (S )‐ and (R )‐configured epoxides are obtained in generally excellent yields (up to 98%) and with good to high enantioselectivities (up to 89% ee), employing a commercially available system, comprising Takemoto's catalysts and tert‐butyl hydroperoxide (TBHP). Highly reactive new tetrasubtituted alkenes allow the first successful use of Takemoto's catalyst under oxidative conditions. Interestingly, density functional theory studies show that TBHP is exclusively activated by the amino thiourea, with halogen‐bonding interactions playing a critical role in affecting the stereochemical outcome of the epoxidation. Derivatizations provide access to densely functionalized trifluoromethylated compounds, featuring one or two vicinal carbon quaternary stereocenters, with the second center being introduced in a highly stereoselective manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


