通过双向氢平衡系统的无偏置光电化学硝基苯到苯胺的转化
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
开发一种低成本、无污染的哈伯式工艺对现代化学工业具有重要意义。传统的化学工业在恶劣条件下依赖化学计量还原剂,产生大量废物,并存在安全风险。光电化学(PEC)氢化是一种利用水和太阳能的有前途的替代方法。然而,在硝基苯制苯胺(NB-to-AN)等六电子/六质子还原过程中,其实现面临两个主要障碍:严重的载流子重组和低效率的氢供应。为了克服这些问题,我们提出了一种“双向氢平衡系统”,该系统集成了缺铜CuBi2O4 (Cu1-xBi2O4)光电阴极(通过Cu空位增强电荷分离)和BiVO4光阳极进行肼氧化反应(HzOR)。HzOR同时降低了系统能量需求并为阴极还原提供质子。串联装置在1个太阳照射下实现了1.23 mA/cm2的光电流密度,无外部偏压,在15小时后保持了88.6%的活性,并提供了接近定量的NB转换和AN选择性(~ 100%),证明了一种无污染,高效的PEC方法用于haber型工艺。本文章由计算机程序翻译,如有差异,请以英文原文为准。
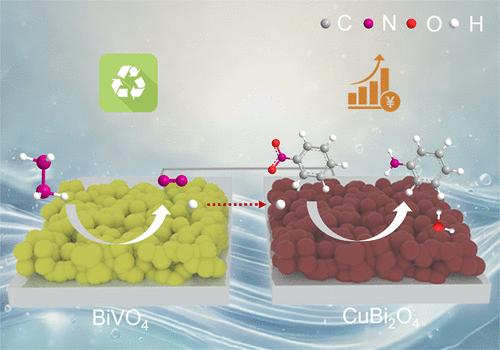
Bias-Free Photoelectrochemical Nitrobenzene-to-Aniline Conversion via a Bidirectional Hydrogen Balance System
Developing a low-cost and pollution-free Haber-type process has importance for the modern chemical industry, which traditionally relies on stoichiometric reductants under harsh conditions, generating significant waste and posing safety risks. Photoelectrochemical (PEC) hydrogenation offers a promising alternative using water and solar energy. However, its implementation for six-electron/six-proton reduction, such as nitrobenzene to aniline (NB-to-AN), faces two major hurdles: severe charge carrier recombination and inefficient hydrogen provision. To overcome these problems, we propose a “bidirectional hydrogen balance system” integrating a Cu-deficient CuBi2O4 (Cu1–xBi2O4) photocathode (enhancing charge separation via Cu vacancies) with a BiVO4 photoanode performing hydrazine oxidation reaction (HzOR). The HzOR simultaneously lowers the system energy demand and supplies protons for cathodic reduction. The tandem device achieved a photocurrent density of 1.23 mA/cm2 without external bias under 1 sun illumination, retained 88.6% activity after 15 h, and delivered near-quantitative NB conversion and AN selectivity (∼100%), demonstrating a pollution-free, efficient PEC approach for Haber-type processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


