球磨炭黑促进Fenton氧化机理及不同氧官能团的定量动力学研究
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
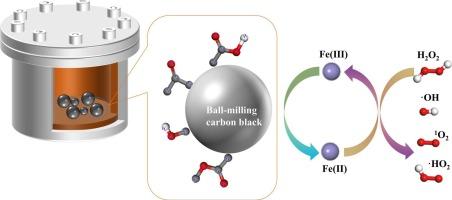
Fenton法中Fe2+的再生利用了碳材料的催化性能,提高了污染物的去除效率,但纯碳材料的催化位点有限,改性条件复杂且成本高。本文通过简单的球磨处理得到更多的含氧官能团,这些官能团将Fe3+吸附到表面进行反应,加速了Fe2+的再生,明显增强了炭黑的催化效果。经过5 h、7 h、9 h和11 h的球磨处理,炭黑的AO7去除率分别为83 %、68 %、74 %和77 %。通过对Fe3+还原实验的动力学研究,定量计算出含氧官能团COOH、COOC、COH和CO的二级动力学速率分别为0.00235 ~ 0.00288 µM−1·min−1、0.00061 ~ 0.00086 µM−1·min−1、0.00017 ~ 0.00034 µM−1·min−1、0.006 ~ 0.00659 µM−1·min−1。此外,用相同的方法对小孔活性炭进行动力学分析也得到了类似的结果。结果表明,这种简单的球磨方法可用于在其他纯碳材料上添加活性位点,并可用于确定材料表面含氧官能团还原Fe3+的具体反应方程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanism of Fenton oxidation promoted by ball-milled carbon black and quantitative kinetic study of different oxygen functional groups
Regeneration of Fe2+ in the Fenton process using the catalytic properties of carbon materials to improve pollutant removal efficiency, but pure carbon materials have limited catalytic sites, and the modification conditions are complicated and costly. In this paper, more oxygen-containing functional groups were obtained by simple ball-milling treatment, and these functional groups adsorbed Fe3+ to the surface for reaction, accelerated the regeneration of Fe2+, and obviously enhanced the catalytic effect of carbon black. After 5 h, 7 h, 9 h, and 11 h of ball milling treatment to carbon black, the AO7 removal rates were 83 %, 68 %, 74 %, and 77 %, respectively. Through kinetic studies of Fe3+ reduction experiments, the secondary kinetic rates for oxygen-containing functional groups COOH, COOC, COH, and CO were quantitatively calculated as 0.00235–0.00288 µM−1·min−1, 0.00061-0.00086 µM−1·min−1, 0.00017–0.00034 µM−1·min−1, 0.006–0.00659 µM−1·min−1. Furthermore, kinetic analysis of small-pore activated carbon using the same method yielded similar results. It has been shown that this simple ball-milling method is useful for the addition of active sites to other pure carbon materials, as well as for determining the specific reaction equation for the reduction of Fe3+ by oxygen-containing functional groups on the surface of the material in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


