硫化氢在氧化还原稳态和程序性细胞死亡中的调节:癌症的机制见解和意义
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
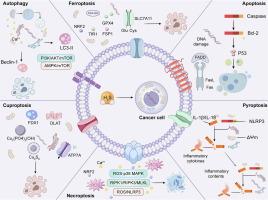
硫化氢(H2S)是一种由含硫氨基酸代谢产生的关键内源性气体介质,在癌症中表现出浓度依赖的二元性。在生理浓度下,H2S通过激活NRF2/Keap1通路,抑制脂质过氧化作用发挥抗氧化作用,从而促进肿瘤细胞存活。相反,超生理水平的H2S通过破坏线粒体稳态、触发活性氧(ROS)爆发和通过过硫化修饰关键蛋白质来诱导程序性细胞死亡(PCD)。H2S和PCD通路之间复杂的相互作用凸显了其作为治疗靶点的潜力,新兴的策略侧重于调节内源性H2S的产生和开发靶向H2S释放化合物。目前的药理学方法包括抑制H2S合成酶和外源性给药H2S供体,这些方法在临床前模型和临床试验中显示出克服治疗耐药和提高治疗效果的希望。这篇综述建立了H2S生物学与六种不同的PCD模式的整合框架,重点关注其在癌症治疗中的潜在应用。它进一步研究了h2s疗法临床转化中的核心挑战,特别是实现靶向递送和管理浓度依赖效应的双重障碍。为了克服这些挑战,我们概述了新兴的转化策略,利用酶靶向抑制剂,重新利用FDA已批准的H2S调节药物,并整合能够刺激触发和空间精确释放H2S的新型纳米治疗平台。未来的研究应优先开发具有精确时空控制的智能递送系统,并破译h2s介导的PCD的动态调控,这对于推进这些机制深入了解精确肿瘤治疗至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrogen sulfide regulation in redox homeostasis and programmed cell death: mechanistic insights and implications in cancer
Hydrogen sulfide (H2S), a key endogenous gaseous mediator derived from sulfur-containing amino acid metabolism, exhibits a concentration-dependent duality in cancer. At physiological concentrations, H2S exerts antioxidant effects by activating the NRF2/Keap1 pathway and suppressing lipid peroxidation, thereby promoting tumor cell survival. In contrast, supraphysiological levels of H2S induce programmed cell death (PCD) by impairing mitochondrial homeostasis, triggering reactive oxygen species (ROS) bursts, and modifying critical proteins via persulfidation. The complex interplay between H2S and PCD pathways highlights its potential as a therapeutic target, with emerging strategies focusing on modulating endogenous H2S production and developing targeted H2S-releasing compounds. Current pharmacological approaches include inhibiting H2S-synthesizing enzymes and exogenous administration of H2S donors, which have shown promise in preclinical models and clinical trials for overcoming therapy resistance and enhancing treatment efficacy. This review establishes the integrative framework bridging H2S biology with six distinct PCD modalities, focusing on its potential therapeutic applications in cancer therapy. It further investigates the core challenges in clinical translation of H2S-based therapies, particularly the dual hurdles of achieving targeted delivery and managing concentration-dependent effects. To overcome these challenges, we outline emerging translational strategies that leverage enzyme-targeted inhibitors, repurpose H2S-modulating drugs already approved by the FDA, and integrate novel nano-theranostic platforms capable of stimulus-triggered and spatially precise release of H2S. Future research should prioritize developing intelligent delivery systems with precise spatiotemporal control, and deciphering the dynamic regulation of H2S-mediated PCD, which will be essential for advancing these mechanistic insights into precision oncology therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


