桉木屑热解动力学分析与数值模拟
IF 6.2
1区 农林科学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
摘要
采用热重分析法(TGA)研究了在氮气气氛(99.99 %,30 mL/min)下,桉木屑在升温速率为5、10、15、20和30℃/min(20 ~ 900℃)下的热分解特性和动力学参数。将桉木屑热解过程分为干燥预热(DH)、快速失重热解(FP)和碳生长(CG)三个阶段。机理函数拟合确定DH和FP阶段分别服从n级反应和jander型三维扩散。DH和FP阶段的活化能E、指数前因子对数lgA和反应阶数n分别为82.42和143.40 kJ/mol、10.77和9.00 s−1和2.58和2.00。在转化率(α) 0.2 ~ 0.8范围内,结果与用FWO和Friedman方法估算的E和lgA值高度一致。在CG阶段,通过对α、温度(T)和升温速率(β)的数值模拟,建立了新的反应过程拟合方程(α=0.8695β0.1319lnT−5.0275β0.1442α=0.8695β0.1319lnT−5.0275β0.1442)。较高的决定系数(R2>0.9700)证实了方程的可靠性。该研究为桉树木屑热解工艺优化和反应器设计提供了重要的理论支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
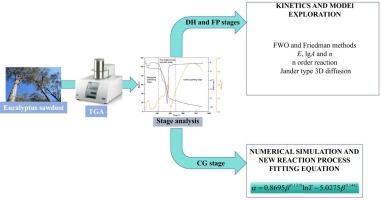
Kinetics analysis and numerical simulation of eucalyptus sawdust pyrolysis
In this study, thermogravimetric analysis (TGA) was employed to investigate the thermal decomposition characteristics and kinetic parameters of eucalyptus sawdust at heating rates of 5, 10, 15, 20, and 30 ℃/min (20–900℃) under a nitrogen atmosphere (99.99 %, 30 mL/min). The pyrolysis process of eucalyptus sawdust was divided into three stages: drying and preheating (DH), fast weight-losing pyrolysis (FP) and carbon growing (CG). Mechanism function fitting determined that the DH and FP stages followed follow n-order reaction and Jander-type 3D diffusion, respectively. The activation energy (E), pre-exponential factor logarithm (lgA) and reaction order (n) of the DH and FP stages are 82.42 and 143.40 kJ/mol, 10.77 and 9.00 s−1, and 2.58 and 2.00, respectively. The results are highly consistent with the E and lgA values estimated by FWO and Friedman methods in the conversion rate (α) range of 0.2–0.8. For the CG stage, a new reaction process fitting equation () was developed by numerical simulation of fitting α, temperature (T) and heating rate (β). The high coefficient of determination (R2>0.9700) confirms the equation's reliability. This study provides crucial theoretical support for optimizing the pyrolysis process of eucalyptus sawdust and guiding the reactor design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial Crops and Products
农林科学-农业工程
CiteScore
9.50
自引率
8.50%
发文量
1518
审稿时长
43 days
期刊介绍:
Industrial Crops and Products is an International Journal publishing academic and industrial research on industrial (defined as non-food/non-feed) crops and products. Papers concern both crop-oriented and bio-based materials from crops-oriented research, and should be of interest to an international audience, hypothesis driven, and where comparisons are made statistics performed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


