双活性中心吩噻嗪阴极与乙腈溶剂化调制协同制备高性能有机锌电池
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
摘要
p型有机材料是一种很有前途的电极材料,但由于活性中心部分在整个结构中的比例较低(通常小于100 mAh g−1),其容量受到限制。为了克服这一挑战,本研究提出了基于吩噻嗪(PTZ)分子的双活性中心氧化还原体系。DFT计算表明,PTZ的芳构性在逐步氧化过程中得到增强,π共轭体系的扩展不仅增强了结构稳定性,而且有利于快速氧化还原反应,其核无关化学位移(NICS(1)zz)值较小,能隙差减小。为了突破动力学限制,引入乙腈(ACN)对溶剂化环境进行了精确调节。第一性原理模拟表明,这种添加剂将内部溶剂化鞘层重组为更松散的结构,降低了脱溶剂能垒,并实现了显著的速率性能。组装的Zn||PTZ电池在0.05 a g−1时具有126 mAh g−1的高比容量,并且在5 a g−1下具有优异的稳定性,在10,000次循环中不会退化。值得注意的是,当电流密度从0.1到10 A g−1增加100倍时,系统保持了80.1%的容量保持,与纯水系统相比,提高了10.8%。最终实现了134.58 Wh kg - 1的能量密度和6201.67 W kg - 1的功率密度。此外,Zn||PTZ电池在-20°C下的容量保持率为75.9%,显示出卓越的低温耐受性。更重要的是,即使在12 mg cm−2的高质量载荷条件下,它仍然表现出优异的传质和动力学稳定性。最后,通过一系列原位和非原位表征,对PTZ电极的多步可逆储能机理进行了深入的研究和讨论。本文章由计算机程序翻译,如有差异,请以英文原文为准。
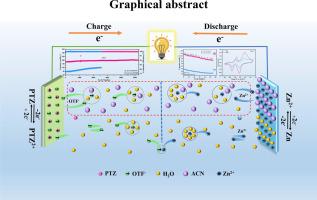
Dual active center phenothiazine cathode synergized with acetonitrile solvation modulation for high performance zinc organic battery
P-type organic materials are promising electrode materials, but their capacity is limited owing to the low proportion of active center parts within the whole structures (usually less than 100 mAh g−1). To overcome this challenge, this study proposes a dual active center redox system based on phenothiazine (PTZ) molecule. DFT calculation show that the aromaticity of PTZ is enhanced during stepwise oxidation, and the extension of its π-conjugated system not only enhances structural stability but also facilitates rapid redox reactions, as evidenced by the smaller Nuclear Independent Chemical Shift (NICS(1)zz) value and reduced energy gap difference. To breakthrough kinetic limitations, the solvation environment was precisely modulated by acetonitrile (ACN) introduction. First-principles simulations reveal this additive restructures the inner solvation sheath into a looser configuration, reducing the de-solvation energy barrier and enabling remarkable rate performance. The assembled Zn||PTZ battery achieve a high specific capacity of 126 mAh g−1 at 0.05 A g−1 and exhibit excellent stability without degradation for 10,000 cycles at 5 A g−1. Notably,the system maintaining 80.1% capacity retention when the current density was scaled up 100-fold from 0.1 to 10 A g−1, representing a 10.8% enhancement compared to pure aqueous systems. It ultimately achieved an energy density of 134.58 Wh kg−1 and a power density of 6201.67 W kg−1. Additionally, the Zn||PTZ battery exhibits 75.9% capacity retention at -20°C, indicating remarkable low temperature tolerance. More importantly, even under high mass loading conditions of 12 mg cm−2, it still demonstrates excellent mass transfer and kinetic stability. Finally, the multi-step reversible energy storage mechanism of PTZ electrodes was thoroughly investigated and discussed via a series of in-situ and ex-situ characterizations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


