二维相关拉曼光谱揭示了-NH2驱动的3-氨基丙醇-水体系氢键网络的动态重构
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
利用自发和受激拉曼光谱(SRS)、二维相关拉曼光谱(2D Raman- cos)和密度泛函数理论(DFT)计算系统地研究了3-氨基丙醇(3AP) -水混合物中的氢键(HB)网络动力学和分子间相互作用。浓度相关的拉曼光谱分析显示,−OH、−NH2和−CH2拉伸模式具有明显的振动位移,且在3AP体积分数为0.3和0.7时呈现非线性趋势和临界跃迁。二维Raman-COS显示HB网络优先进行- NH2重组,其次是- CH2和水OH扰动,表明了顺序响应机制。DFT计算阐明了3AP-(H2O)2、3AP-H2O和(3AP)2 -H2O簇的稳定构型,证明了3AP-水体系中O - h:N/O HB的竞争性协同作用,验证了三阶段溶剂化机制的分子基础。本研究为两亲分子对溶剂微环境的动态调控提供了分子水平的见解,为理解溶液体系中HB网络重组提供了新的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
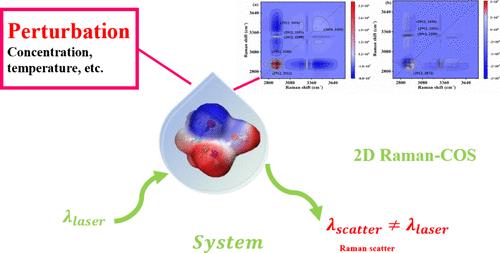
–NH2 Driven Dynamic Reconstruction of Hydrogen-Bond Networks in 3-Aminopropanol–Water System Revealed by Two-Dimensional Correlation Raman Spectroscopy
The hydrogen-bond (HB) network dynamics and intermolecular interactions in 3-aminopropanol (3AP)–water mixtures were systematically investigated using spontaneous and stimulated Raman spectroscopy (SRS), two-dimensional correlation Raman spectroscopy (2D Raman-COS), and density functional theory (DFT) calculations. Concentration-dependent Raman spectral analyses revealed distinct vibrational shifts in −OH, −NH2, and −CH2 stretching modes, with nonlinear trends and critical transitions at 3AP volume fractions of 0.3 and 0.7. 2D Raman-COS demonstrated preferential −NH2 restructuring of the HB network, followed by −CH2 and water OH perturbations, indicating a sequential response mechanism. DFT calculations elucidated the stable configurations of the 3AP-(H2O)2, 3AP-H2O, and (3AP)2–H2O clusters, demonstrating the competitive O–H:N/O HB synergy in the 3AP–water system and validating the molecular basis of the three-stage solvation mechanism. This study provides molecular-level insights into the dynamic regulation of solvent microenvironments by amphiphilic molecules, offering a new perspective for understanding HB network reorganization in solution systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


