乳酸化:通过与慢性炎症的相互作用治疗肌肉骨骼疾病的一个有希望的靶点
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
肌肉骨骼疾病(MSDs)包括影响骨骼、关节和肌肉的疾病,导致严重的疼痛和功能损害,是一个主要的全球健康问题。在MSDs中,关键的代谢过程,如缺氧条件下的糖酵解和乳酸积累,会破坏细胞能量稳态。乳酸化是一种由过量乳酸引起的翻译后修饰,可以修饰组蛋白和非组蛋白,并与炎症和MSD发病机制有关。临床前研究表明,靶向乳酸化有望治疗各种MSDs。系统性、慢性、低度炎症(SCLGI)被认为与许多msd的发病和持续有关,其未解决的状态驱动慢性疾病进展。促进scgi的消退可能会减轻症状,特别是通过来自膳食必需多不饱和脂肪酸(PUFAs)的专门促消退介质(SPMs)。SPMs及其小分子类似物在炎症相关疾病(包括关节病、骨质疏松症和肌肉萎缩症)的动物模型中均显示出治疗效果。这篇综述提出了一种新的MSDs治疗策略,将乳酸化抑制剂或激动剂与促进scgi解决的药物结合起来。这种综合方法可以提高MSD管理的有效性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
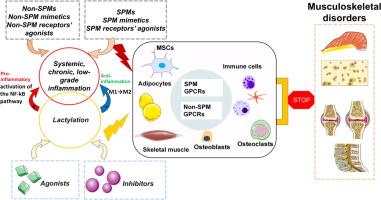
Lactylation: a promising target for musculoskeletal disorders via interactions with chronic inflammation
Musculoskeletal disorders (MSDs) encompass conditions that affect bones, joints, and muscles, leading to substantial pain and functional impairment and representing a major global health concern. In MSDs, key metabolic processes—such as glycolysis and lactate accumulation under hypoxic conditions—disrupt cellular energy homeostasis. Lactylation, a post-translational modification arising from excessive lactate, modifies both histone and non-histone proteins and has been implicated in inflammation and MSD pathogenesis. Preclinical studies indicate that targeting lactylation holds promise for the treatment of various MSDs. Systemic, chronic, low-grade inflammation (SCLGI) is thought to contribute to both the onset and persistence of many MSDs, with its unresolved state driving chronic disease progression. Promoting the resolution of SCLGI may alleviate symptoms, particularly through specialized pro-resolving mediators (SPMs) derived from dietary essential polyunsaturated fatty acids (PUFAs). Both SPMs and their small-molecule analogs have demonstrated therapeutic benefits in animal models of inflammation-related disorders, including arthropathies, osteoporosis, and muscular dystrophy.This review proposes a novel therapeutic strategy for MSDs that integrates lactylation inhibitors or agonists with agents that facilitate SCLGI resolution. Such a combined approach may enhance the effectiveness of MSD management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


