骨钙素的可塑性和与Gprc6A共定位的增强可能有助于达斡尔地松鼠冬眠时肌肉骨骼的维持。
IF 2.2
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology
Pub Date : 2025-10-04
DOI:10.1016/j.cbpa.2025.111941
引用次数: 0
摘要
目的:冬眠哺乳动物为研究废用诱导的肌肉骨骼变性的抵抗机制提供了一个自然模型。然而,在长时间不运动的情况下,使肌肉和骨骼保存的分子途径仍然不够明确。本研究检测了达斡尔地松鼠(达乌尔鼠)在冬眠状态下骨钙素及其受体G蛋白偶联受体家族C组6成员A (Gprc6A)的时间动态,以阐明冬眠觉醒周期中肌肉骨骼维持的潜在机制。方法:在冬眠的关键阶段,定量评估后肢肌肉和骨量以及胫骨骨微观结构。采用免疫印迹法和免疫荧光共定位法检测血清骨钙素浓度、骨钙素和Gprc6A的表达谱。结果:肌肉骨骼质量和胫骨微观结构在冬眠期间只有微小的变化(P > 0.05)。与夏季活跃期相比,冬眠期间胫骨骨钙素表达明显下降,但在间歇觉醒期间恢复。在比目鱼肌(SOL)和指长伸肌(EDL)中,骨钙素的表达在间歇唤醒时明显上调(P 结论:达斡尔地松鼠在长时间冬眠中表现出显著的肌肉骨骼弹性,尽管长时间不活动,但仍能保持结构完整性。在觉醒阶段骨钙素和Gprc6A的振荡表达,结合在睡眠期间持续的配体-受体共定位,可能构成了一种协调的内分泌-肌肉-骨骼适应,在冬眠期间保持组织功能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
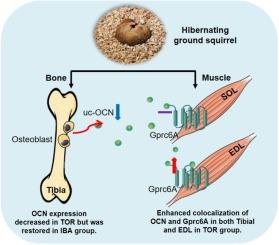
Osteocalcin plasticity and enhanced colocalization with Gprc6A may contribute to musculoskeletal maintenance in hibernating Daurian ground squirrels
Objectives
Hibernating mammals provide a natural model for investigating mechanisms of resistance to disuse-induced musculoskeletal degeneration. However, the molecular pathways enabling muscle and bone preservation during prolonged inactivity remain insufficiently defined. This study examined the temporal dynamics of osteocalcin and its receptor G protein-coupled receptor family C group 6 member A (Gprc6A) across hibernation states in Daurian ground squirrels (Spermophilus dauricus) to elucidate potential mechanisms of musculoskeletal maintenance during torpor-arousal cycles.
Methods
Hindlimb muscle and bone mass, along with tibial bone microstructure, were quantitatively assessed at key hibernation phases. Serum osteocalcin concentrations and expression profiles of osteocalcin and Gprc6A were evaluated by western blotting and immunofluorescent colocalization analyses.
Results
Musculoskeletal mass and tibial microstructure exhibited only minor changes across hibernation periods (P > 0.05). Tibial osteocalcin expression decreased significantly during torpor compared to the summer active phase but was restored during interbout arousal. In the soleus (SOL) and extensor digitorum longus (EDL) muscles, osteocalcin expression was markedly up-regulated during interbout arousal relative to torpor (P < 0.05). Gprc6A expression in the EDL also increased significantly during interbout arousal compared to summer and torpor phases. Enhanced colocalization of osteocalcin and Gprc6A was observed in both tibial bone and EDL muscle during torpor (P < 0.05).
Conclusion
Daurian ground squirrels exhibited remarkable musculoskeletal resilience during prolonged hibernation, maintaining structural integrity despite extended inactivity. Oscillatory expression of osteocalcin and Gprc6A during arousal phases, combined with sustained ligand-receptor colocalization during torpor, may constitute a coordinated endocrine-musculoskeletal adaptation that preserves tissue function during hibernation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.00
自引率
4.30%
发文量
155
审稿时长
3 months
期刊介绍:
Part A: Molecular & Integrative Physiology of Comparative Biochemistry and Physiology. This journal covers molecular, cellular, integrative, and ecological physiology. Topics include bioenergetics, circulation, development, excretion, ion regulation, endocrinology, neurobiology, nutrition, respiration, and thermal biology. Study on regulatory mechanisms at any level of organization such as signal transduction and cellular interaction and control of behavior are also published.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


