雌性和雄性小鼠早期海马突触前gaba能终端的差异:急性早期炎症的影响
IF 4
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
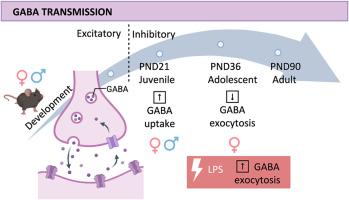
GABA决定突触连接的效率,影响其发育的复杂性,但其作用受发育性别差异的调节,而发育性别差异影响其神经支配的效率。我们研究了雄性和雌性小鼠在幼年期(PND21)、青春期(PND36)和成年期(PND90)海马末端GABA储存和胞外分泌的效率机制。分析突触前GABA转运蛋白1型(GAT1)和囊状GABA转运蛋白VGAT1的mRNA表达。在PND21,直到成年,两性都检测到GAT1 mRNA水平(SLC6A1)的显著下降,而SLC32A1-VGAT mRNA水平则保持不变。我们还分析了GAT1和VGAT蛋白的密度。Western blot分析揭示了GAT1的单体和寡聚形式的存在。在两性的不同发育阶段,单体形态的密度是保守的。不同的是,在PND21雄性和雌性小鼠的海马突触体溶解物中,低聚物组装显著过表达,但在PND36时恢复。与成体颗粒相比,PND21和PND36雄性海马突触体溶解物中的VGAT密度基本保守,但PND21雌性颗粒中的VGAT密度明显降低。值得注意的是,这些变化是一致的,并支持PND21雄性和雌性海马突触体中新摄取的[3H]GABA的囊泡储存改变,以及GABA能雄性和雌性突触体对增加的去极化刺激(12、20和30 mM氯化钾富集溶液)的不同反应性(以[3H]GABA胞分泌效率衡量)。有趣的是,急性LPS治疗以性别依赖的方式影响PND36的GABA胞吐效率。这些结果为GABA在发育早期中枢抑制性可塑性的作用及其在生理病理条件下二形适应的相关性提供了新的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Differences in presynaptic hippocampal GABAergic terminals at the early stage of life in female and male mice: effect of an acute early inflammatory challenge
GABA dictates the efficiency of synaptic connection, influencing its developmental complexity, but its role is tuned by developmental sex differences which affect the efficiency of its innervation. We investigated the efficiency of mechanisms of GABA storage and exocytosis in hippocampal terminals of male and female mice during the juvenile period (PND21), adolescence (PND36) or adulthood (PND90). The expression of mRNA encoding for the presynaptic GABA transporter type 1, (GAT1) and the vesicular GABA transporter (VGAT1) was analysed. A significant scaling-down in the GAT1 mRNA levels (SLC6A1) was detected at PND21 in both sexes until adulthood, while the SLC32A1-VGAT mRNA level was conserved. We also analysed the density of GAT1 and VGAT proteins. Western blot analysis unveiled the presence of a monomeric and an oligomeric form of GAT1. The density of the monomeric form was conserved at the different stages of development in both sexes. Differently, the oligomeric assembly was significantly overexpressed in hippocampal synaptosomal lysates from PND21 male and female mice, but recovered at PND36. VGAT density was largely conserved in PND21 and PND36 male hippocampal synaptosomal lysates when compared to adult particles, but significantly lower in PND21 female particles. Notably, these changes are consistent and support the altered vesicular storage of newly taken-up [3H]GABA detected in PND21 male and female hippocampal synaptosomes as well as the different responsiveness of GABAergic male and female synaptosomes to increasing depolarizing stimuli (12, 20 and 30 mM KCl-enriched solutions) measured as efficiency of the [3H]GABA exocytosis. Interstingly, an acute LPS treatment affects the efficiency of GABA exocytosis at PND36 in a sex-dependent manner. These results add new knowledge on the role of GABA as effector of central inhibitory plasticity at the early stage of development and its relevance in dimorphic adaptation in physio pathological conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neurochemistry international
医学-神经科学
CiteScore
8.40
自引率
2.40%
发文量
128
审稿时长
37 days
期刊介绍:
Neurochemistry International is devoted to the rapid publication of outstanding original articles and timely reviews in neurochemistry. Manuscripts on a broad range of topics will be considered, including molecular and cellular neurochemistry, neuropharmacology and genetic aspects of CNS function, neuroimmunology, metabolism as well as the neurochemistry of neurological and psychiatric disorders of the CNS.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


