nbs诱导天然单萜酮-香芹酮和-马鞭草酮在乙腈中的氧化磺化反应
IF 0.8
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究了nbs诱导羰基萜类化合物l-香芹酮和l-马鞭草酮在乙腈中的氧化磺化反应。左旋香芹酮与多种磺胺类化合物的一锅反应可得到产率高达74%的2,4-二甲基-1-磺酰基咪唑啉类香芹酮衍生物和副产物9-溴香芹酮。在l-马尾草酮的反应中,得到了2,4,4,7-四甲基- 4h -苯并[e][1,3]恶嗪-3-溴化铵盐,与所取的磺胺无关,并通过单晶x射线分析证实了其结构。提出了两种反应的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
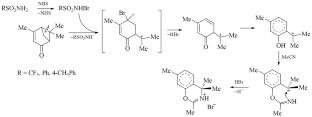
The NBS-Induced Oxidative Sulfonamidation of Natural Monoterpene Ketones l-Carvone and l-Verbenone in Acetonitrile
NBS-Induced oxidative sulfonamidation of carbonyl terpenoids l-carvone and l-verbenone in acetonitrile was studied. The one-pot reaction of l-carvone with various sulfonamides affords 2,4-dimethyl-1-sulfonylimidazoline carvone derivatives in up to 74% yield and 9-bromocarvone as a by-product. In the reaction of l-verbenone, 2,4,4,7-tetramethyl-4H-benzo[e][1,3]oxazin-3-ium bromide salt was obtained irrespective of the taken sulfonamide, and its structure was proved by single crystal X-ray analysis. The mechanisms of both reactions were proposed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


