al修饰的七嗪基g-C3N4纳米管吸附氢的DFT研究
IF 1.8
4区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
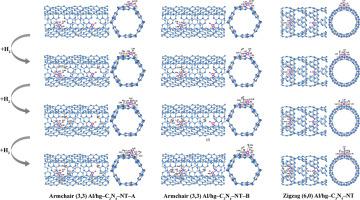
采用周期DFT方法研究了两种不同类型的七嗪基石墨氮化碳纳米管(hg-C3N4-NTs),即扶手状(3,3)和锯齿状(6,0)的Al原子修饰的hg-C3N4-NTs对多个氢分子的吸附。第一个氢分子吸附在al修饰的hg-C3N4-NTs的所有外表面,被发现是解离的H2化学吸附,并表现出比随后的氢分子(第二到第四)更强的相互作用。值得注意的是,第一个氢分子吸附在扶手椅(3,3)和锯齿形(6,0)hg-C3N4-NT上,表现出较高的储氢潜力,其中锯齿形(6,0)hg-C3N4-NT的化学吸附最强,吸附能为-1.89 eV。此外,相应的原始扶手椅(3,3)和之字形(6,0)可以作为hg-C3N4-NTs的代表性分子模型。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A DFT investigation of hydrogen adsorption onto the Al-decorated heptazine-based g-C3N4 nanotubes
The adsorption of multiple hydrogen molecules on two different types of heptazine-based graphitic carbon nitride nanotubes (hg‒C3N4‒NTs), namely armchair (3,3) and zigzag (6,0) hg‒C3N4‒NTs, decorated with Al atom, was investigated using the periodic DFT method. The first hydrogen molecule adsorbed on all outer surfaces of Al-decorated hg‒C3N4‒NTs was found to be a dissociative H2 chemisorption and exhibited significantly stronger interaction than subsequent hydrogen molecules (the second to fourth). Notably, the first hydrogen molecule adsorbed on Al-decorated on armchair (3,3) and zigzag (6,0) hg‒C3N4‒NTs demonstrated high potential for hydrogen storage, with the strongest chemisorption observed on Al-decorated zigzag (6,0) hg‒C3N4‒NT, which exhibited an adsorption energy of -1.89 eV. Furthermore, the corresponding pristine armchair (3,3) and zigzag (6,0) hg‒C3N4‒NTs can serve as representative molecular models for the hg‒C3N4‒NTs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Surface Science
化学-物理:凝聚态物理
CiteScore
3.30
自引率
5.30%
发文量
137
审稿时长
25 days
期刊介绍:
Surface Science is devoted to elucidating the fundamental aspects of chemistry and physics occurring at a wide range of surfaces and interfaces and to disseminating this knowledge fast. The journal welcomes a broad spectrum of topics, including but not limited to:
• model systems (e.g. in Ultra High Vacuum) under well-controlled reactive conditions
• nanoscale science and engineering, including manipulation of matter at the atomic/molecular scale and assembly phenomena
• reactivity of surfaces as related to various applied areas including heterogeneous catalysis, chemistry at electrified interfaces, and semiconductors functionalization
• phenomena at interfaces relevant to energy storage and conversion, and fuels production and utilization
• surface reactivity for environmental protection and pollution remediation
• interactions at surfaces of soft matter, including polymers and biomaterials.
Both experimental and theoretical work, including modeling, is within the scope of the journal. Work published in Surface Science reaches a wide readership, from chemistry and physics to biology and materials science and engineering, providing an excellent forum for cross-fertilization of ideas and broad dissemination of scientific discoveries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


