自由基反合成:现代萜类天然产物组装的有力策略
IF 39
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
激进的反合成已经成为复杂天然产物的现代组装的一种强大策略,它提供了一种单电子逻辑,补充了传统的极性断开。这篇综述强调了最近的进展,特别是在过去十年中发展起来的激进方法及其在萜类天然产物全合成中的战略性应用。重点放在各种自由基前体,包括烯烃、羧酸、卤化物、醇、羰基化合物和烷烃,是如何通过创新的过程转化的,如光氧化还原催化、金属氢化物氢原子转移(MHAT)、氧化还原活性酯(RAE)化学、交叉亲电偶联(XEC)和基于hat的C-H功能化。通过组织对前体类和相应反应模式的讨论,我们说明了基于自由基的合成策略如何能够有效地构建四元立体中心和复杂多环支架的模块化组装。本文章由计算机程序翻译,如有差异,请以英文原文为准。
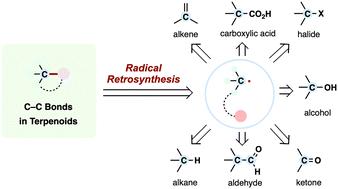
Radical retrosynthesis: a powerful strategy for modern assembly of terpenoid natural products
Radical retrosynthesis has emerged as a powerful strategy for the modern assembly of complex natural products, offering a one-electron logic that complements traditional polar disconnections. This review highlights recent advances—particularly developed over the past decade—in radical methodologies and their strategic applications in the total synthesis of terpenoid natural products. Emphasis is placed on how various radical precursors, including alkenes, carboxylic acids, halides, alcohols, carbonyl compounds, and alkanes, have been transformed through innovative processes such as photoredox catalysis, metal–hydride hydrogen atom transfer (MHAT), redox-active ester (RAE) chemistry, cross-electrophile couplings (XEC), and HAT-based C–H functionalization. By organizing the discussion around precursor classes and corresponding reaction modes, we illustrate how radical-based synthetic strategies enable efficient construction of quaternary stereocenters and modular assembly of complex polycyclic scaffolds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Society Reviews
化学-化学综合
CiteScore
80.80
自引率
1.10%
发文量
345
审稿时长
6.0 months
期刊介绍:
Chemical Society Reviews is published by: Royal Society of Chemistry.
Focus: Review articles on topics of current interest in chemistry;
Predecessors: Quarterly Reviews, Chemical Society (1947–1971);
Current title: Since 1971;
Impact factor: 60.615 (2021);
Themed issues: Occasional themed issues on new and emerging areas of research in the chemical sciences
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


