有机合成中的亚砜基碳烯和亚砜基亚烯
IF 39
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
亚砜基羰基和亚砜基亚烯是两亲性中间体,在其反应中心附近有一个未氧化的硫原子。它们独特的性质和可调节的反应活性使它们成为研究环加成反应、原子掺入、后期官能化等应用中的羰基和亚硝基的模型物种。它们最近获得了极大的关注,并为新反应的开发和发现带来了可观的希望。本文分析了亚砜基甲苯和亚砜基甲苯的化学性质,重点介绍了它们的生成及其在现代有机合成中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
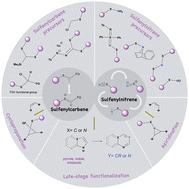
Sulfenylcarbenes and sulfenylnitrenes in organic synthesis
Sulfenylcarbenes and sulfenylnitrenes are ambiphilic intermediates possessing an unoxidized sulfur atom adjacent to their reactive center. Their unique properties and tunable reactivity make them a model species for studying carbenes and nitrenes in cycloaddition reactions, atom incorporation, late-stage functionalizations, among other applications. They have gained significant attention recently and hold considerable promise for novel reaction development and discovery. Herein, we analyze the chemistry of sulfenylcarbenes and sulfenylnitrenes, emphasizing their generation and applications in contemporary organic synthesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Society Reviews
化学-化学综合
CiteScore
80.80
自引率
1.10%
发文量
345
审稿时长
6.0 months
期刊介绍:
Chemical Society Reviews is published by: Royal Society of Chemistry.
Focus: Review articles on topics of current interest in chemistry;
Predecessors: Quarterly Reviews, Chemical Society (1947–1971);
Current title: Since 1971;
Impact factor: 60.615 (2021);
Themed issues: Occasional themed issues on new and emerging areas of research in the chemical sciences
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


