2,8-二芳基-1,5-萘啶嘧啶作为恶性疟原虫磷脂酰肌醇4-激酶抑制剂的优化研究
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
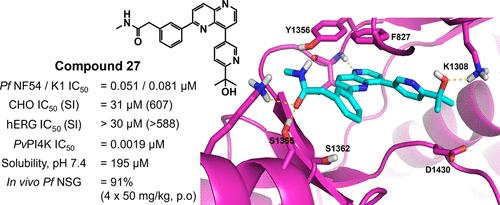
先前报道的抗疟疟原虫磷脂酰肌醇4-激酶IIIβ 2,8-二芳基-1,5-萘啶抑制剂显示出次优的物理化学和药代动力学性质。基于靶标的结构-活性关系和结构-性能优化研究发现了几种具有良好靶活性和全细胞活性以及改善的理化性质的化合物。在人源化NOD-scid il - 2r - γ小鼠模型中,一种新的领先化合物27在4 × 50 mg/kg QD剂量下显示出改善的药代动力学特征和降低寄生虫血症(91%)。化合物27对hERG通道没有高浓度抑制作用,对哺乳动物没有细胞毒性,但对相关的人脂质激酶(PI3Kα和PI4Kβ)具有低选择性;然而,对人类MINK1和MAP4K4激酶观察到明显更高的选择性边际。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Optimization of 2,8-Diaryl-1,5-naphthyridines as Plasmodium falciparum Phosphatidylinositol 4-Kinase Inhibitors with Improved ADME Profiles and In Vivo Efficacy
Previously reported antimalarial Plasmodium phosphatidylinositol 4-kinase IIIβ 2,8-diaryl-1,5-naphthyridine inhibitors have shown suboptimal physicochemical and pharmacokinetic properties. A focused target-based structure–activity relationship and structure–property optimization studies identified several compounds with good target and whole-cell activities and improved physicochemical properties. A new frontrunner compound 27 showed an improved pharmacokinetic profile and reduced parasitaemia (91% at 4 × 50 mg/kg QD doses) in the humanized NOD-scid IL-2Rγnull mouse model of Plasmodium falciparum malaria. Compound 27 poses no hERG channel inhibition at high concentrations or mammalian cytotoxicity but shows low selectivity against related human lipid kinases (PI3Kα and PI4Kβ); however, significantly higher selectivity margins were observed against the human MINK1 and MAP4K4 kinases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


