带有siRNA和辛伐他汀的两性离子超分子纳米组件协同治疗动脉粥样硬化
IF 12.1
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
动脉粥样硬化易损斑块含有各种不良成分,包括过量的胆固醇、efferocytodeficient巨噬细胞和炎症因子。然而,传统的降脂药物由于无法针对复杂的病理生理机制,往往表现出有限的疗效。在此,创新的两性离子超分子纳米组件,称为PCDSC‐S2P,被开发为纳米清除剂,以减少和稳定斑块。纳米组件是基于两性离子聚(羧甜菜碱)修饰的β -环糊精(β - CD)作为宿主分子,包封辛伐他汀(Sim)作为客体,并静电吸收靶向钙/钙调素依赖性蛋白激酶IIγ (siCaMKIIγ)的小干扰RNA。PCDSC - S2P纳米组件由主客体相互作用、静电相互作用和氢键驱动。此外,巨噬细胞靶向肽修饰指导纳米组装体向动脉粥样硬化斑块积聚。因此,PCDSC - S2P作为纳米清除剂,可以通过β - CD介导的胆固醇去除、Sim -驱动的脂质降低和sicamkii - γ增强的efferocytosis协同作用于病变斑块。因此,PCDSC - S2P治疗可调节炎症细胞因子的释放并表现出抗炎活性。此外,PCDSC - S2P可以显著减少高脂饮食中载脂蛋白E缺乏小鼠的斑块面积并稳定斑块。总之,PCDSC‐S2P在小干扰RNA制剂的开发中显示出巨大的潜力,显示出其在动脉粥样硬化治疗中的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
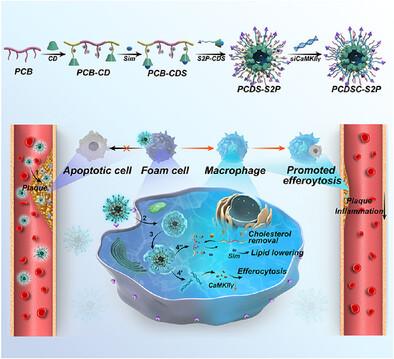
Zwitterionic Supramolecular Nanoassemblies With siRNA and Simvastatin as “Nanoscavenger” for Synergistic Atherosclerosis Treatment
Atherosclerotic vulnerable plaques contain various undesirable components, including excess cholesterol, efferocytosis‐deficient macrophages, and inflammatory factors. However, conventional lipid‐lowering drugs often show limited efficacy due to their inability to target the complex pathophysiology. Herein, innovative zwitterionic supramolecular nanoassemblies, termed PCDSC‐S2P, are developed as the nanoscavenger to reduce and stabilize plaques. The nanoassemblies are based on zwitterionic poly(carboxybetaine)‐modified β‐cyclodextrin (β‐CD) acting as a host molecule that encapsulates simvastatin (Sim) as a guest and absorbs small interfering RNA targeting calcium/calmodulin‐dependent protein kinase II gamma (siCaMKIIγ) electrostatically. The PCDSC‐S2P nanoassemblies are driven by host‐guest interactions, electrostatic interactions, and hydrogen bonds. Moreover, the macrophage‐targeting peptide modification directs nanoassemblies accumulation toward atherosclerotic plaques. Therefore, PCDSC‐S2P, as the nanoscavenger, can act at the lesional plaque via β‐CD‐mediated cholesterol removal, Sim‐driven lipid lowering, and siCaMKIIγ‐enhanced efferocytosis synergistically. Consequently, the PCDSC‐S2P treatment regulates the release of inflammatory cytokines and exhibits anti‐inflammatory activity. Furthermore, PCDSC‐S2P can significantly reduce plaque area and stabilize plaques in apolipoprotein E‐deficient mice fed with a high‐fat diet. Together, PCDSC‐S2P shows great potential as a new delivery system in the development of small interfering RNA formulation, showing its promise for atherosclerosis treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


