氯离子阻滞对碱性海水持久氧化的界面静电工程
IF 12.1
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
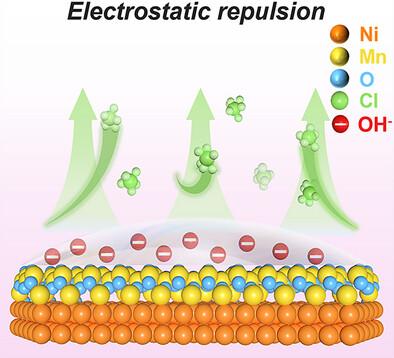
海水电解中Cl−的存在削弱了析氧反应(OER)通过析氯反应(CER)的选择性,并导致氯点蚀导致材料失效。引入阴离子层不仅可以阻止Cl -的跃迁,而且不会阻碍含氧阴离子的扩散,而且可以在外表面原位生成。在这里,通过界面静电工程,开发了一种分层催化剂,该催化剂由形成在Ni泡沫衬底上的MnO2层组成,该衬底上均匀覆盖着镍-铁层状双氢氧化物(NiFe - LDH)活性层。研究发现,引入的Lewis酸层(MnO2)可以在表面自发生成OH -基团,并通过静电斥力排斥Cl -。同时,锚定在MnO2基体内部的Ni活性位点可以改善MnO2的低活性。多层NiFe‐LDH@MnO2/NF阳极可以在70°C下,在100 mA cm−2的电流密度下稳定工作100小时,并保持97.9%的OER选择性。此外,OER过电位降低了136 mV,优于工业环境中最先进的商用Ni网,特别是该设备可以由风能供电。本研究为设计低成本、高稳定性的高性能海水电解提供了一种有效的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Interfacial Electrostatic Engineering for Chlorine Ions Blocking Toward Long‐Lasting Alkaline Seawater Oxidation
The existence of Cl− in seawater electrolysis weakens the selectivity of the oxygen evolution reaction (OER) via the chlorine evolution reaction (CER) and causes material failure by chlorine pitting. Introducing anion layers not only repels the transition of Cl− without retarding the diffusion of oxygen‐containing anions but also can be generated in situ on the outer surface. Here, a hierarchical catalyst consisting of a MnO2 layer formed on a Ni foam substrate covered uniformly by a nickel‐iron layered double hydroxide (NiFe‐LDH) active layer is developed through interfacial electrostatic engineering. It was found that the introduced Lewis acid layer (MnO2 ) can spontaneously generate OH− groups on the outer surface in situ to repel Cl− by electrostatic repulsion force. Meanwhile, Ni‐active sites anchored inside the MnO2 matrix can improve the low activity of MnO2 . The multilayer NiFe‐LDH@MnO2 /NF anode can operate steadily at the current density of 100 mA cm−2 at 70 °C for 100 h and maintain 97.9% OER selectivity. Furthermore, the OER overpotential was reduced by 136 mV, superior to the state‐of‐the‐art commercial Ni mesh in industrial environments, especially the device can be powered by wind energy. The work offers an efficient strategy for designing high‐performance seawater electrolysis with low cost and high stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


