扫描电化学电池显微镜观察非晶碳薄膜电极阴离子插入-萃取反应对钒(IV/V)反应的影响
IF 12.1
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
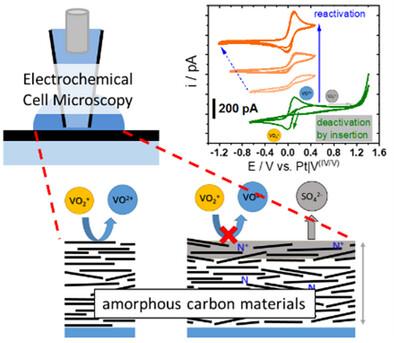
采用扫描电化学电池显微镜(SECCM)模拟全钒氧化还原液流电池(VRFB)正极的反应,探讨了高电位对厚度为9 ~ 30 nm无氮和掺氮的非晶态氢化碳薄膜电极对钒(IV/V)氧化还原反应的影响。除了从循环伏安图(CV)中评估峰分离(EPP)外,还采用了局部探测方法,以分析高过电位对碳材料稳定性的影响以及竞争电化学过程。硫酸盐阴离子插入过程是所有样品的主要过程,其开始与钒(IV/V)反应平行。吡啶/吡咯基团的存在使插入物稳定,对钒(IV/V)反应的抑制作用更强。在所有情况下,钒(IV/V)反应的电化学氧化还原特征,以及碳薄膜的初始拉曼光谱,都可以通过在适当的时间范围内施加还原电位来完全重建,即使在极化到非常高的电位(2.5 V vs. RHE)之后。总的来说,在讨论钒(IV/V)氧化还原反应的电化学数据时,必须更多地考虑这种竞争性插入反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The Role of the Anion Insertion-Extraction Reaction in Amorphous Carbon Thin Film Electrodes on the Vanadium(IV/V) Reaction Probed by Scanning Electrochemical Cell Microscopy
The influence of high potentials on amorphous nitrogen-free and nitrogen-doped hydrogenated carbon thin film electrodes with thicknesses of 9 to 30 nm is probed toward the vanadium(IV/V) redox reaction by scanning electrochemical cell microscopy (SECCM), which mimics the reaction of the positive side of the all-vanadium redox flow battery (VRFB). Besides the evaluation of the peak separation (EPP) from cyclic voltammograms (CV), the localized probing is adapted in a way that the influence of high overpotentials on the stability of the carbon materials, as well as competitive electrochemical processes, can be analyzed. The sulfate anion insertion process is found to be the predominant process in all samples, with its onset appearing in parallel to the vanadium(IV/V) reaction. The presence of pyridine/pyrrole groups can stabilize the insertion compound, which inhibits the vanadium(IV/V) reaction much more strongly. In all cases, the electrochemical redox features of the vanadium(IV/V) reaction, as well as the initial Raman spectra of the carbon thin films, are fully reconstructed by applying reductive potentials in a suitable time frame, even after polarizing to drastically high potentials (2.5 V vs. RHE). Overall, this competing insertion reaction must be given greater consideration when discussing electrochemical data of the vanadium(IV/V) redox reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


