PiP‐Plex:一种用于单细胞分泌蛋白质的多路定量的粒子中粒子系统
IF 26.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
细胞信号是由各种蛋白质的分泌调节的,这些蛋白质可以用来推断细胞的表型。然而,在单细胞水平上,用常用的方法很难检测到这些蛋白。在这里,我们提出了PiP - plex颗粒-颗粒(PiP)系统,用于共聚焦显微镜下的多重蛋白分泌分析。PiP复合物由(i)荧光强度条形码微颗粒(bmp)与(ii)海藻酸盐水凝胶颗粒内的单个细胞共包裹。我们发现,PiPs维持了90%的细胞活力,并允许活细胞回收。在PiPs中建立了七重荧光条形码和伴随的三明治免疫分析,检测限从0.8 pg mL - 1到2 ng mL - 1,取决于蛋白质。PiP - plex测定法以批量免疫测定法为基准,并发现与它们竞争或优于它们。通过PiP - plex检测暴露于脂多糖的单个THP - 1细胞分泌的蛋白质,并检测到不同的细胞反应,包括MIP - 1α、TNF - α和IL - 17A的显著增加;MIP‐1α和IL‐17A是最常分泌的细胞因子,而其他细胞因子通常是共分泌的。使用PiP - plex,我们分析了约750个THP - 1细胞,展示了其在表征细胞和基于细胞的治疗方法(如癌症免疫治疗)方面的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
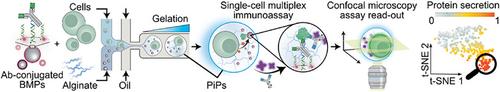
PiP‐Plex: A Particle‐in‐Particle System for Multiplexed Quantification of Proteins Secreted by Single Cells
Cell signaling is modulated by the secretion of various proteins, which can be used to infer a cell's phenotype. However, these proteins cannot be readily detected in multiplex by commonly used methods at the single‐cell level. Here we present PiP‐plex a particles‐in‐particle (PiPs) syst for multiplex protein secretion analysis by confocal microscopy. PiP‐plex‐comprises (i) fluorescence intensity barcoded microparticles (BMPs) co‐entrapped with (ii) a single cell inside an alginate hydrogel particle. We found that PiPs maintained >90% cellular viability and allowed live cells retrieval. A seven‐plex fluorescent barcoding and concomitant sandwich immunoassay in PiPs was developed with limits of detection ranging from 0.8 pg mL−1 to 2 ng mL−1 depending on the protein. PiP‐plex assays were benchmarked with bulk immunoassays and found to rival or outperform them. Proteins secreted by single THP‐1 cells upon exposure to lipopolysaccharid were measured by PiP‐plex and varying cell responses detected, including a significant increase in MIP‐1α, TNF‐α, and IL‐17A; MIP‐1α and IL‐17A were the most frequently secreted cytokines, while other cytokines were typically co‐secreted. Using PiP‐plex, we analyzed ≈750 THP‐1 cells, showcasing its potential for characterizing cells and cell‐based therapeutics for e.g. cancer immunotherapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


