过渡金属氰酰胺Li2MnSn2(NCN)的拓扑转变
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
用NO2BF4和n-BuLi分别作为化学氧化剂和还原剂,研究了Li在Li2MnSn2(NCN)6中的可逆(脱)插层作用。这些反应是拓扑定向的,当NCN2 -支架倾斜时,脱嵌Li2-xMnSn2 (NCN)6 (x = 0-1.7)保留P3 1m结构,以适应Li空位的引入,同时当Mn2+被氧化成Mn4+时,Mn-N键长度也随之收缩。SQUID磁强计也证实了这种氧化,表明顺磁矩减少,XANES测量也表明,在Li脱嵌时Mn - k边缘位置明显向高能量移动。采用电化学方法在有机电解液中研究了Li2MnSn2(NCN)6在锂离子电池材料中的初步应用。循环伏安测量结果显示,Mn2+/Mn3+和Mn3+/Mn4+氧化还原对具有两对阳极峰和两对阴极峰。这些结果为过渡金属碳二亚胺的插层化学研究提供了第一个实验指示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
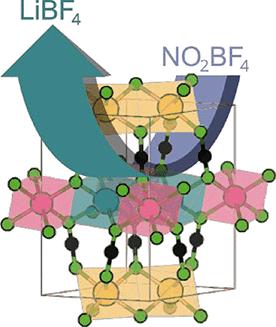
Topotactic Transformation of the Transition-Metal Cyanamide Li2MnSn2(NCN)6
The reversible (de)intercalation of Li from Li2MnSn2(NCN)6 has been demonstrated using NO2BF4 and n-BuLi as chemical oxidation and reduction agents, respectively. These reactions are topotactic with deintercalated Li2–xMnSn2(NCN)6 (x = 0–1.7) retaining the structure as the NCN2– scaffold tilts such as to accommodate the introduction of Li vacancies, together with a concomitant contraction of the Mn–N bond length while Mn2+ is oxidized toward Mn4+. This oxidation is also confirmed from SQUID magnetometry which evidences a decrease in the paramagnetic moment and, also, from XANES measurements showing a clear shift of the Mn K-edge position to higher energies upon Li deintercalation. The tentative application of Li2MnSn2(NCN)6 in Li-ion battery materials was also investigated using electrochemical methods in an organic electrolyte. Cyclic voltammetry measurements reveal two pairs of anodic and cathodic peaks which are assigned to Mn2+/Mn3+ and the Mn3+/Mn4+ redox-pairs. These results provide the first experimental indication toward the as yet untapped intercalation chemistry of transition-metal carbodiimides.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


