碳酸氢铵的热分解
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
碳酸氢铵(NH4HCO3)的热分解由于其释放气态产物而不留下固体残留物的能力,在许多工业和技术应用中都是相关的。这项工作提出了一个全面的动力学和形态学研究,其分解使用非等温差示扫描量热法(DSC)和互补表征技术。采用Friedman等转换方法对多个加热方案进行了分析,并结合动力学分析建立了预测动力学模型。结果表明,分解从~ 75°C开始通过一个吸热步骤进行,释放出NH3、CO2和H2O。得到的动力学参数为表观活化能为101 kJ/mol, ln(a / s-1) = 28.1。截断后的Šesták-Berggren动力学模型为(1 - α)0.54α0.17,表明该反应机制以成核和生长为主。部分分解颗粒的扫描电镜证实了形态演化与所提出的动力学途径一致。经过验证的模型准确地再现了实验结果,并成功地预测了超出实验范围的分解行为,证明了其鲁棒性和潜在的工业用途。本文章由计算机程序翻译,如有差异,请以英文原文为准。
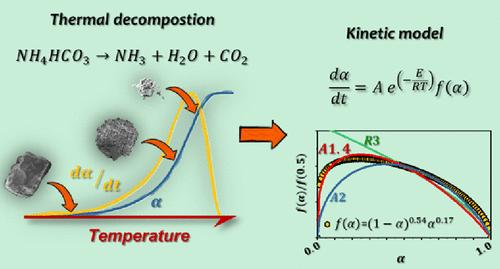
Thermal Decomposition of Ammonium Bicarbonate
The thermal decomposition of ammonium bicarbonate (NH4HCO3) is relevant in numerous industrial and technological applications due to its ability to release gaseous products without leaving solid residues. This work presents a comprehensive kinetic and morphological study of its decomposition using nonisothermal differential scanning calorimetry (DSC) and complementary characterization techniques. Multiple heating programs were analyzed using the Friedman isoconversional method and combined kinetic analysis to build a predictive kinetic model. The results indicate that decomposition proceeds from ∼75 °C through a single endothermic step, releasing NH3, CO2, and H2O. The kinetic parameters obtained were an apparent activation energy of 101 kJ/mol and a ln(A/s–1) = 28.1. The optimized truncated Šesták–Berggren kinetic model, (1 – α)0.54α0.17, suggests a mechanism dominated by nucleation and growth. Scanning electron microscopy of partially decomposed particles confirmed the morphological evolution consistent with the proposed kinetic pathway. The verified model accurately reproduced experimental results and successfully predicted decomposition behavior under conditions beyond the experimental range, demonstrating its robustness and potential usefulness for industrial use.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


