从分子动力学模拟的角度理解烯烃在聚乙烯中的溶解度
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
研究了乙烯(ETH)和1-己烯(HEX)在聚乙烯(PE)中在80℃和不同压力下的吸附机理。模拟结果表明,ETH分子可以阻碍HEX-PE相互作用,减少HEX与PE链的聚类。这可能解释了实验中观察到的ETH对HEX的抗溶剂作用。相反,HEX可以作为载体增强ETH的吸附,促进ETH与PE链端的相互作用。这种共溶性效应是通过引入一个校正项Sindirect来捕获的,该校正项解释了ETH分子通过HEX间接与PE相互作用。因此,ETH的总溶解度表示为SETH = Sdirect + γ sindirect,其中Sdirect对应ETH直接与PE相互作用,γ代表ETH分子同时与HEX和PE相互作用的贡献。我们的研究结果表明,MD为复杂的多组分系统(如聚烯烃反应器中遇到的系统)的分子水平吸附机制提供了强有力的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
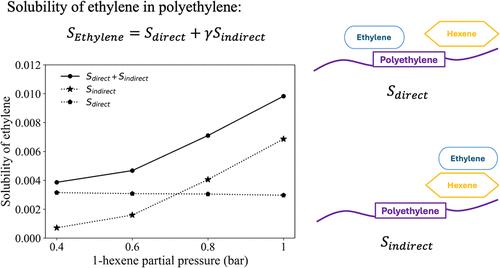
Understanding Olefin Solubility in Polyethylene from a Molecular Dynamics Simulation Perspective
We investigated the sorption mechanisms of ethylene (ETH) and 1-hexene (HEX) in polyethylene (PE) using molecular dynamics (MD) simulations at 80 °C and various pressures. The simulations revealed that ETH molecules can hinder HEX-PE interactions, reducing HEX clustering with PE chains. This may explain the experimentally observed antisolvent effect of ETH on HEX. Conversely, HEX can enhance the sorption of ETH by acting as carriers, facilitating ETH interactions with PE chain ends. This cosolubility effect was captured by introducing a correction term, Sindirect, which accounts for ETH molecules that interact indirectly with PE via HEX. Total ETH solubility is thus expressed as SETH = Sdirect + γSindirect, where Sdirect corresponds to ETH directly interacting with PE and γ represents the contribution from ETH molecules interacting simultaneously with HEX and PE. Our findings demonstrate that MD offer powerful insights into the molecular-level mechanisms governing sorption in complex, multicomponent systems, such as those encountered in polyolefin reactors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


