离子对动力学导致碳电极上双电层的非线性电化学结构
IF 17.1
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
双电层(EDL)的压电效应对离子浓度的非线性趋势已被广泛观察到,但其原子尺度的起源尚不清楚。在本研究中,在不同离子浓度的LiCl电解质中,对破坏极化亥姆霍兹平面的多壁碳纳米管(MWCNT)能量收集器进行了离子配对解锁。低浓度(≤2m)的LiCl电解质使Li +和Cl⁻形成独立的水合结构,形成具有清晰极化亥姆霍兹平面、定义明确的EDL。在高浓度(>2 M)下,氢键水网络被削弱,导致离子团簇之间的静电相互作用更强。这导致Li(H2O)4-Cl(H2O)8离子对的形成,其中Li +与Cl⁻部分共享其水配体。这种离子配对破坏了Li +在MWCNT表面的球内吸附,从而降解了EDL结构。这些结果表明,过量的离子配对使EDL中的空间电荷分布均匀化,从而减弱了压电离子效应。这些发现为edl作为离子浓度函数的结构演变提供了原子见解,并为优化基于mwcnts的能量存储和收集装置提供了重要指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
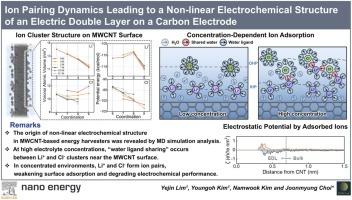
Ion pairing dynamics leading to a non-linear electrochemical structure of an electric double layer on a carbon electrode
A non-linear trend in piezoionic effects of an electric double layer (EDL) on ion concentration has been widely observed experimentally, yet its atomic-scale origin remains unclear. In this study, ion pairing that destroy polarized Helmholtz planes were unlocked for multi-walled carbon nanotube (MWCNT) energy harvesters with different ion concentrations of LiCl electrolyte. LiCl electrolyte with low concentrations (≤2 M) allowed Li⁺ and Cl⁻ to form independently hydrated structures, facilitating a well-defined EDL with clearly polarized Helmholtz planes. At high concentrations (>2 M), the hydrogen-bonded water network was weakened, leading to stronger electrostatic interactions between ion clusters. This resulted in the formation of Li(H2O)4-Cl(H2O)8 ion pairs, where Li⁺ partially shared its water ligand with Cl⁻. Such ion pairing disrupted inner-sphere adsorption of Li⁺ onto the MWCNT surface, thereby degrading the EDL structure. These results indicate that excessive ion pairing homogenize the spatial charge distributions in the EDL, thereby weakening the piezoionic effects. These findings provide atomic insights into the structural evolution of EDLs as a function of ion concentration, and offer critical guidelines for optimizing MWCNT-based energy storage and harvesting devices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


