道路锰氧化物和干湿循环驱动下轮胎磨损颗粒中6PPD向6PPD- q的加速转变:界面催化与气候应力耦合
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
本研究揭示了道路锰氧化物和干湿循环加速轮胎磨损颗粒(TWPs)中N-(1,3-二甲基丁基)-N ' -苯二胺(6PPD)氧化为有毒的6PPD-醌(6PPD- q)的协同机制。三种Mn加载策略(Mn 2 +吸附、MnOx包覆、原位δ-MnO 2合成)加上模拟干湿循环(12 h循环:10 h干燥 + 2 h雨淋)表明,原位MnOx加载的TWPs (4.2 mg MnOx/g)达到了最高的6PPD-Q产率(3.48 mg/L),符合s型动力学(R² = 0.999)。主要机制包括:1)湿相:Mn(III)(↑650%)和水膜中溶解有机物(³DOM*)的三激发态介导O₂•毒化,攻击6PPD形成6PPD- oo•,并通过质子耦合电子转移(PCET)转化为6PPD- q;2)干相:环境持久性自由基(EPFRs)积累(g因子漂移:2.0031至2.0041)和预激活的Mn位点(Mn(III)*↑90.29%);3)重湿相:溶解的Mn(II)/Fe²⁺引发fenton样反应,生成•OH(5.0 × 10⁹自旋/μL)氧化6PPD。清除剂实验证实ROS(•OH/O₂•毒血症)是Mn催化的关键桥梁(6PPD-Q产率↓90%)。富里酸(FA)衍生物通过醌络合作用将6PPD-Q的半衰期延长至53.7 h,增强了环境持久性。本研究为6PPD-Q的来源控制提供了气候矿物调节策略,强调了管理道路含锰材料和干湿循环影响以减轻水生生态系统风险的迫切需要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
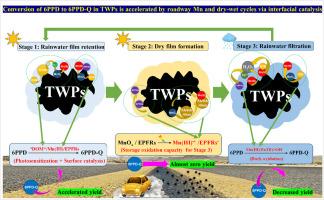
Accelerated Transformation of 6PPD to 6PPD-Q in Tire Wear Particles Driven by Roadway Manganese Oxides and Dry-Wet Cycles: Interfacial Catalysis Coupled with Climatic Stressors
This study reveals the synergistic mechanism whereby roadway manganese oxides and dry-wet cycling accelerate N-(1,3-dimethylbutyl)-N’-phenylenediamine (6PPD) oxidation to toxic 6PPD-quinone (6PPD-Q) in tire wear particles (TWPs). Three Mn-loading strategies (Mn²⁺ adsorption, MnOx coating, in-situ δ-MnO₂ synthesis) coupled with simulated dry-wet cycles (12 h cycle: 10 h drying + 2 h rain spray) demonstrated that in-situ MnOx-loaded TWPs (4.2 mg MnOx/g) achieved the highest 6PPD-Q yield (3.48 mg/L), following sigmoidal kinetics (R² = 0.999). Key mechanisms include: 1) Wet phase: Mn(III) (↑650%) and triple excited states of dissolved organic matter (³DOM*) in water films mediated O₂•⁻ generation, attacking 6PPD to form 6PPD-OO•, which converted to 6PPD-Q via proton-coupled electron transfer (PCET); 2) Dry phase: Environmental Persistent Free Radicals (EPFRs) accumulated (g-factor shift: 2.0031 to 2.0041) and pre-activated Mn sites (Mn(III)* ↑ 90.29%); 3) Rewetting phase: Dissolved Mn(II)/Fe²⁺ triggered Fenton-like reactions, generating •OH (5.0 × 10⁹ spins/μL) to oxidize 6PPD. Scavenger experiments confirmed ROS (•OH/O₂•⁻) as critical bridges for Mn catalysis (6PPD-Q yield ↓ 90%). Fulvic acid (FA) derivatives prolonged 6PPD-Q half-life to 53.7 h via quinone complexation, enhancing environmental persistence. This study provides a climate-mineral regulation strategy for source control of 6PPD-Q, highlighting the critical need to manage roadway Mn-containing materials and dry-wet cycling impacts to mitigate aquatic ecosystem risks.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


