仿生永生化间充质干细胞纳米颗粒通过CD73靶向和化疗抑制原位术后胶质瘤
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
胶质瘤术后极易复发,缺乏有效的术后辅助治疗药物。开发能够有效穿过血脑屏障并靶向术后胶质瘤的药物对于克服胶质瘤治疗相关的挑战至关重要。用细胞膜修饰的纳米材料已经显示出跨越血脑屏障治疗胶质瘤的希望,但是细胞的来源以及它们的异质性是目前这种策略的瓶颈。先前,我们证明了永生化间充质干细胞膜保留了天然的肿瘤归巢能力,并为持续的肿瘤靶向提供了稳定、可扩展和统一的来源。在这里,我们提出使用具有肿瘤归家特性的永生化间充质干细胞质膜作为载体,开发装载shCD73和阿霉素的仿生纳米颗粒,命名为Lipo-PM@shCD73@DOX。利用肿瘤归巢因子,制备的仿生纳米颗粒能有效穿过血脑屏障,靶向胶质瘤术后残留组织,实现胶质瘤术后基因治疗和化疗。本研究证实了仿生纳米颗粒通过抑制细胞增殖和诱导细胞凋亡来延缓胶质瘤的进展。此外,我们还证实了仿生纳米颗粒的体外和体内生物安全性。总之,本研究克服了以往模拟策略中细胞来源不足和细胞异质性的问题,开发了抗胶质瘤靶向药物仿生纳米颗粒,有效地穿过血脑屏障。它构成了一种新的抗胶质瘤佐剂,增强术后治疗和延迟复发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
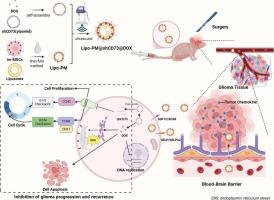
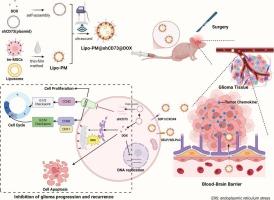
Biomimetic immortalized mesenchymal stem cell-based nanoparticles suppress orthotopic postsurgical glioma via CD73 targeting and chemotherapy
Glioma is highly prone to recurrence post-surgery, and effective postoperative adjuvant therapeutic agents are lacking. Developing drugs that can efficiently cross the blood-brain barrier and target postoperative glioma is crucial for overcoming the challenges associated with the treatment of glioma. Nanomaterials modified with cell membranes have shown promise in crossing the blood-brain barrier for the treatment of glioma, but the origin of the cells as well as their heterogeneity are the current bottlenecks of this strategy. Previously, we demonstrated that immortalized mesenchymal stem cell membranes retain natural tumor-homing capability and offer a stable, scalable, and uniform source for sustained tumor targeting. Here, we proposed the use of immortalized mesenchymal stem cell plasma membranes with tumor-homing properties as carriers to develop biomimetic nanoparticles loaded with shCD73 and doxorubicin, named Lipo-PM@shCD73@DOX. By harnessing the tumor-homing factors, the developed biomimetic nanoparticles effectively crossed the blood-brain barrier and targeted postoperative residual glioma tissues to achieve postoperative gene therapy and chemotherapy for glioma. The present study demonstrates the therapeutic efficacy of biomimetic nanoparticles in delaying glioma progression by inhibiting cellular proliferation and inducing apoptosis. Additionally, we confirmed the in vitro and in vivo biosafety of biomimetic nanoparticles. In conclusion, this study overcame the problems of insufficient cell source and cellular heterogeneity in previous mimetic strategies and developed anti-glioma targeting drug biomimetic nanoparticles that efficiently cross the blood-brain barrier. It constitutes a novel anti-glioma adjuvant that enhances postoperative therapy and delays recurrence.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


